- Lenalidomide
-

- $0.00 / 25Kg/Bag
-
2024-04-22
- CAS:191732-72-6
- Min. Order: 2Kg/Bag
- Purity: 98% up, USP / BP
- Supply Ability: 20tons
- Lenalidomide
-
- $8.00 / 1KG
-
2024-03-29
- CAS:191732-72-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Lenalidomide
-

- $0.00 / 1KG
-
2024-03-16
- CAS:191732-72-6
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
|
| | Lenalidomide Basic information |
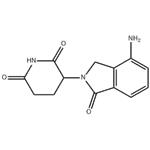
| Product Name: | Lenalidomide | | Synonyms: | 3-(4-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione,Lenalidomide;LenalidoMide(CC-5013,RevliMid);Lenalidomide ,98%;(3S)-3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione;(3R)-3-(4-aMino-1-oxo-2,3-dihydro-1H-isoindol-2-yl)piperidine-2,6-dione;LenalidoMide (RevliMid);LenalidoMide-d5;3-(4-AMino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione | | CAS: | 191732-72-6 | | MF: | C13H13N3O3 | | MW: | 259.26 | | EINECS: | 691-297-1 | | Product Categories: | Amines;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Inhibitor;CC-5013;Inhibitors;Molecular Targeted Antineoplastic;pharmaceutical intermediate;API;191732-72-6 | | Mol File: | 191732-72-6.mol | 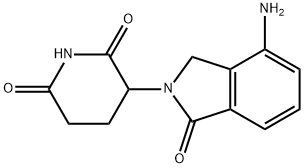 |
| | Lenalidomide Chemical Properties |
| Melting point | 265-268 °C | | Boiling point | 614.0±55.0 °C(Predicted) | | density | 1.460±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | Soluble in DMSO (up to 30 mg/ml) | | pka | 10.75±0.40(Predicted) | | form | solid | | color | White | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | | InChI | InChI=1S/C13H13N3O3/c14-9-3-1-2-7-8(9)6-16(13(7)19)10-4-5-11(17)15-12(10)18/h1-3,10H,4-6,14H2,(H,15,17,18) | | InChIKey | GOTYRUGSSMKFNF-UHFFFAOYSA-N | | SMILES | N1C(=O)CCC(N2CC3=C(C2=O)C=CC=C3N)C1=O | | CAS DataBase Reference | 191732-72-6(CAS DataBase Reference) |
| | Lenalidomide Usage And Synthesis |
| Description | Lenalidomide is a kind of antitumor drugs that developed by American biological pharmaceutical companies. Its chemical structure is similar with thalidomide. It differing in the presence of an amino moiety in the 4-position and removal of one of the carbonyls of the phthaloyl ring. This derivative evolved from a structural-based effort to eliminate the adverse effects (somnolence, neuropathy, and teratogenicity) of thalidomide while maintaining or enhancing the appealing attributes. It has many functions such as anti-tumor, immune regulation and anti-angiogenesis. It can inhibit the secretion of inflammatory cytokines, and increase the secretion of peripheral blood mononuclear anti-inflammatory cytokines. Vitro tests show that lenalidomide can inhibit the hyperplasia of some cell lines such as namalwa cell. It can inhibit the growth of patients’ multiple myeloma cells and MM1S cell. In addition, lenalidomide also can inhibit the expression of oxidase-2 (COX-2), but it has no effect on COX-1. Two multicenter randomized double-blind placebo-controlled clinical studies evaluate the safety and curative effect of lenalidomide that is used for multiple myeloma. The primary efficacy end point of the studies is time to progression (TTP). The interim analysis shows that TTP of the combination group is significantly superior to dexamethasone group. Recent clinical research results show that lenalidomide not only has curative effect on treating MDS and MM, but also on treating myeloma, leukemia, metastatic renal cell carcinoma, solid tumor, idiopathic generalized amyloidosis and systemic bone marrow fibrosis disease with marrow unripe. | | Chemical Properties | Yellow Solid | | Originator | Celgene (US) | | History | In December 2005, the US Food and Drug Administration (FDA) approved lenalidomide to be used in the treatment of myelodysplastic syndrome (MDS).
In March 2006, the FDA approved lenalidomide that was produced by American Celgene Biological Pharmaceutical Companies to be used in the treatment of multiple myeloma (MM).
On September 23, 2011, the European Medicines Agency (EMA) released information that they have confirmed that the benefits of lenalidomide(trade name: Revlimid) to be used in the treatment of patients group that was approved outweighed the risks. Meanwhile they warned the doctor the risk of the drug to cause new cancer cases. Lenalidomide has been used with dexamethasone to treat adult patients with multiple myeloma that has received at least one treatment. Three new studies show that the incidence of new cancer will be increased in the patients with newly diagnosed multiple myeloma treated by lenalidomide and other combined treatment increased incidence of cancer. | | Uses | Lenalidomide is a thalidomide analog known to have immunomodulatory properties. Lenalidomide inhibits TNF-alpha production, stimulates T cells, reduces serum levels of the cytokines vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), and inhibits angiogenesis. This agent also promotes G1 cell cycle arrest and apoptosis of malignant cells. It is used in the therapy of multiple myeloma. | | Uses | Lenalidomide (Revlimid, CC-5013) is a TNF-α secretion inhibitor with IC50 of 13 nM. | | Definition | ChEBI: Lenalidomide is a dicarboximide that consists of 1-oxoisoindoline bearing an amino substituent at position 4 and a 2,6-dioxopiperidin-3-yl group at position 2. Inhibits the secretion of TNF-alpha. It has a role as an angiogenesis inhibitor, an antineoplastic agent and an immunomodulator. It is a member of isoindoles, a dicarboximide, a member of piperidones and an aromatic amine. | | Brand name | Revlimid(Celgene). | | benefits | Lenalidomide is used to treat various types of cancers. It works by slowing or stopping the growth of cancer cells. It is also used to treat anemia in patients with certain blood/bone marrow disorders (myelodysplastic syndromes-MDS). Lenalidomide may lessen the need for blood transfusions. | | Biochem/physiol Actions | Lenalidomide, a derivative of thalidomide, is an immunomodulatory agent that is approved drug for treatment of multiple myeloma. Apparently Lenalidomide is a ligand of ubiquitin E3 ligase cereblon that induces the enzyme to degrade the Ikaros transcription factors IKAROS family zinc finger 1 (IKZF1) and IKZF3. Lenalidomide possess pleiotropic antitumor effects. It is used in the treatment of 5q-deletion associated myelodysplastic syndrome (del(5q)-MDS). | | Mechanism of action | Lenalidomide acts by a novel drug mechanism—modulation of the substrate specificity of the CRL4CRBN E3 ubiquitin ligase. In multiple myeloma, lenalidomide induces the ubiquitination of IKZF1 and IKZF3 by CRL4CRBN. Subsequent proteasomal degradation of these transcription factors kills multiple myeloma cells. | | target | TNF-α | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
clarithromycin.
Antifungals: concentration possibly increased by
itraconazole and ketoconazole.
Calcium channel blockers: concentration possibly
increased by verapamil.
Cardiac glycosides: possibly increases concentration
of digoxin.
Ciclosporin: concentration possibly increased by
ciclosporin. | | storage | Store at -20°C | | Clinical claims and research | Lenalidomide is a new derivative of thalidomide. But its teratogenic toxicity has not found. Its effectiveness is 100 times stronger than thalidomide. According to the result of three clinical trials, lenalidomide is the most effective drug in the treatment of multiple myeloma. More than half of patients can prolong survival time to more than 3 years after taking the drug. In addition, it is also the only effective drugs to treat myelodysplastic syndrome (MDS). Clinical results find that 64% of the patients with MDS need not use blood transfusion after treated by lenalidomide. | | References | 1) Ito et al. (2010), Identification of a primary target of thalidomide teratogenicity; Science, 327 1345
2) Gandhi et al. (2014), Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T-cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN); Br.J. Haematol., 164 811
3) Kronke et al. (2014), Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells; Science, 343 301
4) Lu et al. (2014), The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins; Science., 343 305 |
| | Lenalidomide Preparation Products And Raw materials |
|