| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | iopronic acid Basic information |
| Product Name: | iopronic acid | | Synonyms: | iopronic acid;lopronic acid;lopronic;2-[[2-[3-(Acetylamino)-2,4,6-triiodophenoxy]ethoxy]methyl]butanoic acid;2-[[2-[3-(Acetylamino)-2,4,6-triiodophenoxy]ethoxy]methyl]butyric acid;B-11420;Bilimiro;Bilimiron | | CAS: | 37723-78-7 | | MF: | C15H18I3NO5 | | MW: | 673.02 | | EINECS: | 253-643-6 | | Product Categories: | | | Mol File: | 37723-78-7.mol | 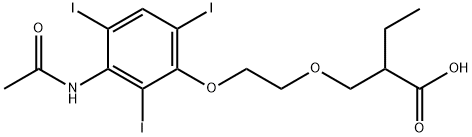 |
| | iopronic acid Chemical Properties |
| Melting point | 130°C | | Boiling point | 674.2±55.0 °C(Predicted) | | density | 2.0615 (estimate) | | pka | 4.28±0.10(Predicted) | | Water Solubility | 20.08g/L(37 ºC) |
| | iopronic acid Usage And Synthesis |
| Originator | Bilimiru,Bracco,Italy,1974 | | Uses | Diagnostic aid (radiopaque medium, cholecystographic). | | Definition | ChEBI: Iopronic acid is an aromatic amide. | | Manufacturing Process | ,A solution of 192 g 3-acetylamino-2,4,6-triiodophenol, sodium (0.35 mol) in
350 ml dimethylacetamide, was mixed with 107.5 g 3-(2-iodoethoxy)-2-
ethylpropionic acid ethyl ester (0.35 mol) at 90°C with stirring over a period
of about 20 to 30 minutes. Stirring was continued while the mixture was held
at 95°C to 100°C for 16 hours. The solvent was then removed by distillation
in a vacuum, and the residue was poured into 4,000 ml water. The solid
precipitate formed was recovered and washed with water, dilute sodium
carbonate solution, dilute sodium bisulfite solution, and again with much
water. The ethyl ester was obtained in a yield of 220 g (90%). When
recrystallized from 75% aqueous ethanol, it melted at 80°C to 86°C.
The ester (70 g, 0.1 mol) was saponified in a boiling mixture of 250 ml
methanol and 250 ml water to which 100 ml N sodium hydroxide solution was
added in small batches with stirring. The methanol was distilled from the
saponification mixture, the residue was mixed with water and extracted with
ethyl acetate. The aqueous phase was acidified with hydrochloric acid in the
presence of sodium bisulfite.
The free acid gradually crystallized from the acidified solution in the amount of42.4 g (63% yield). When recrystallized from 50% ethanol and from ethyl
acetate, it melted at 130°C. | | Brand name | Bilimiro (Bracco
Industria Chimica S.p.A., Italy); Bilimiron (Bracco Industria
Chimica S.p.A., Italy); Oravue (Bristol-Myers
Squibb); Videobil (Bracco Industria Chimica S.p.A.,
Italy). | | Therapeutic Function | Diagnostic aid (radiopaque medium) |
| | iopronic acid Preparation Products And Raw materials |
|