| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | Fenoxedil Basic information |
| Product Name: | Fenoxedil | | Synonyms: | Fenoxedil;2-(p-Butoxyphenoxy)-N-(2,5-diethoxyphenyl)-N-[2-(diethylamino)ethyl]acetamide;Acetamide, 2-(4-butoxyphenoxy)-N-(2,5-diethoxyphenyl)-N-[2-(diethylamino)ethyl]- | | CAS: | 54063-40-0 | | MF: | C28H42N2O5 | | MW: | 486.64 | | EINECS: | | | Product Categories: | | | Mol File: | 54063-40-0.mol | 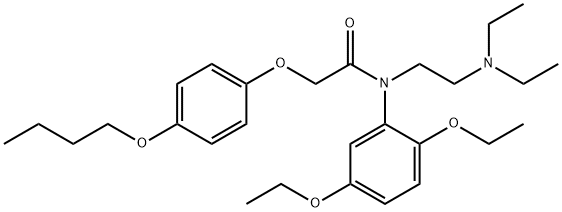 |
| | Fenoxedil Chemical Properties |
| Boiling point | 225-230 °C(Press: 0.1 Torr) | | density | 1.072±0.06 g/cm3(Predicted) | | pka | 8.93±0.10(Predicted) |
| | Fenoxedil Usage And Synthesis |
| Originator | Suplexedil,Hepatrol,France,1974 | | Definition | ChEBI: Fenoxedil is an anilide. | | Manufacturing Process | 420 grams of 2,5-diethoxy aniline are dissolved in 4 liters of dichloroethane
and 230 grams of triethylamine are added. The mixture is heated, while
stirring, with 845 grams of 4-butoxy phenoxy acetyl chloride. The temperature
increases towards 40°C. The mixture is then heated for 2 hours at 80°C. After
cooling the product is washed with normal hydrochloric acid, then with water,
then with normal sodium carbonate and finally with water.
The organic phase is dried over sodium sulfate, filtered, the dichloroethane is
evaporated off and the residue is crystallized from ethyl alcohol (95%). The
product is dried in the oven and there is thus obtained about 800 grams (yield
90%) of the N-(2,5-diethoxyphenyl)-4-butoxy phenoxy acetamide, MP 101°C.
A vessel provided with a mechanical agitator, a thermometer and a
refrigerant, is charged with 49.2 grams of sodamide (90%) in suspension in
300 ml of anhydrous toluene, and a solution of 465 grams of amide obtained
as above in 2 liters of anhydrous toluene. The solution is poured in, little by
little during 1.5 hours with slight warming. The mixture is maintained for 1
hour at 80°C during which ammonia is evolved. It is cooled to 45°C, there is
added, in a single quantity, 170 grams of 2-diethyl-amino-1-chloroethane and
the temperature is raised slowly to 100°C and is maintained there for 10
hours.
The mixture is cooled, the organic phase washed with water and dried over
sodium sulfate. The toluene is evaporated and the residue taken up in 2 liters
of normal acetic acid, with cooling. It is allowed to crystallize in the cold,
filtered to remove the insoluble portion and the base precipitated from the
filtrate by the addition of sodium carbonate; this is extracted with
dichloroethane and the organic phase dried over sodium sulfate. After
evaporation of the solvent an oil is distilled, BP 225° to 230°C/0.1 mm,
weight 340 grams, yield 58%. The hydrochloride prepared by the action of
gaseous hydrogen chloride on this oil in ethyl ether melts at 140°C. | | Therapeutic Function | Vasodilator |
| | Fenoxedil Preparation Products And Raw materials |
|