- Asenapine USP/EP/BP
-
- $1.10 / 1g
-
2021-07-27
- CAS:65576-45-6
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- Asenapine
-

- $15.00 / 1KG
-
2021-07-13
- CAS:65576-45-6
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Asenapine
-

- $15.00 / 1KG
-
2021-07-09
- CAS:65576-45-6
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Asenapine Basic information |
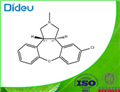
| Product Name: | Asenapine | | Synonyms: | trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrole;Asenapine;(3aR,12bR)-rel-5-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrole;1H-Dibenz(2,3:6,7)oxepino(4,5-C)pyrrole, 5-chloro-2,3,3A,12B-tetrahydro-2-methyl-, (3ar,12br)-rel-;1H-Dibenz(2,3:6,7)oxepino(4,5-C)pyrrole, 5-chloro-2,3,3A,12B-tetrahydro-2-methyl-, trans-;Asenapine [inn:ban];Einecs 265-829-4;Unii-jkz19V908o | | CAS: | 65576-45-6 | | MF: | C17H16ClNO | | MW: | 285.772 | | EINECS: | 2658294 | | Product Categories: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 65576-45-6.mol | 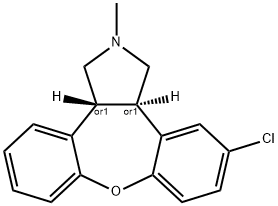 |
| | Asenapine Chemical Properties |
| Boiling point | 357.9±42.0 °C(Predicted) | | density | 1.231 | | storage temp. | Store at +4°C | | solubility | DMSO:50.0(Max Conc. mg/mL);174.97(Max Conc. mM) | | form | A solid | | pka | 9.50±0.20(Predicted) |
| | Asenapine Usage And Synthesis |
| Description | (±)-Asenapine is an atypical antipsychotic. It binds to dopamine D1-4, α-adrenergic, and histamine receptors (Kis = 0.42-1.45, 0.32-1.26, and 1-6.17 nM, respectively), as well as the serotonin (5-HT) receptor subtypes 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, 5-HT6, and 5-HT7 (Kis = 0.03-3.98 nM). (±)-Asenapine inhibits the suppression of neuron firing induced by the 5-HT2A, dopamine D2, and α2-adrenergic receptor agonists 2,5-dimethoxy-4-iodoamphetamine (DOI), apomorphine, and clonidine , respectively, in rat brain (ED50s = 75, 40, and 85 μg/kg, respectively). In vivo, (±)-asenapine (0.05-0.2 mg/kg, s.c.) increases extracellular dopamine levels in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and lateral striatum and suppresses the conditioned avoidance response in rats. It prevents acute and chronic phencyclidine-induced deficits in cued reversal learning in rats when administered at a dose of 0.075 mg/kg. Formulations containing asenapine have been used in the treatment of schizophrenia and bipolar I disorder. | | Chemical Properties | Yellow Oil | | Uses | Combined serotonin (5HT2) and dopamine (D2) receptor antagonist; structurally related to Mianserin. Antipsychotic | | Definition | ChEBI: (R,R)-asenapine is a 5-chloro-2-methyl-2,3,3a,12b-tetrahydrodibenzo[2,3:6,7]oxepino[4,5-c]pyrrole in which both of the stereocentres have R configuration. It is a conjugate base of a (R,R)-asenapine(1+). It is an enantiomer of a (S,S)-asenapine. | | Biological Activity | Novel psychopharmacologic agent. Displays antagonist activity at 5-HT, dopamine, noradrenalin and histamine receptor subtypes (pK i values are 8.60, 8.40, 10.15, 9.75, 10.46, 8.84, 9.60, 9.94, 8.85, 8.90, 8.84, 9.38, 8.95, 8.93, 8.9, 9.49, 8.91, 9.00 and 8.21 for 5-HT 1A , 5-HT 1B , 5-HT 2A , 5-HT 2B , 5-HT 2C , 5-HT 5A , 5-HT 6 , 5-HT 7 , D 1 , D 2L , D 2S , D 3 , D 4 , α 1A , α 2A , α 2B , α 2C , H 1 and H 2 receptors respectively). Displays no appreciable affinity for muscarinic receptors. Exhibits potent activity in animal models predictive of antipsychotic efficacy. | | Clinical Use | Atypical antipsychotic
Treatment of schizophrenia and bipolar disease | | Enzyme inhibitor | This atypical antipsychotic agent (FW = 285.77 g/mol; CAS 65576-45-6), marketed under the trade names Saphris ?, and also known as Org 5222 and (3aRS,12bRS)-rel-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3: 6,7]-oxepino-[4,5-c]pyrrole, is multi-receptor antagonist with the following spectrum of binding interactions: serotonin 5-HT1A receptor, Ki = 2.5 nM; serotonin 5-HT1B receptor, Ki = 4.0 nM; serotonin 5-HT2A receptor, Ki = 0.06 nM; serotonin 5-HT2B receptor, Ki = 0.16 nM; serotonin 5-HT2C receptor, Ki = 0.03 nM; serotonin 5-HT5A receptor, Ki = 1.6 nM; serotonin 5-HT6 receptor, Ki = 1.5 nM; serotonin 5-HT7 receptor, Ki = 0.13 nM; a1- Adrenergic receptor, Ki = 1.2 nM; a2A-Adrenergic receptor, Ki = 1.2 nM; a2B-Adrenergic receptor, Ki = 0.25 nM; a2C-Adrenergic receptor, Ki = 1.2 nM; dopamine D1-receptor, Ki = 1.4 nM; dopamine D2-receptor, Ki = 1.3 nM; dopamine D3-receptor, Ki = 0.4 nM; dopamine D4-receptor, Ki = 1.1 nM; histamine H1-receptor, Ki = 1.0 nM; and histamine H2-receptor, Ki = 6 nM. Like other atypical antipsychotic drugs, asenapine preferentially enhances dopamine and acetylcholine efflux in the rat medial prefrontal cortex and hippocampus. See Reference-x for asenapine’s UV, IR, NMR, and mass spectra as well as X-ray analysis, thermal properties, solubilities and partition coefficient. | | Drug interactions | Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative effects
with opioids.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong the
QT interval; avoid with amiodarone, disopyramide
and procainamide (risk of ventricular arrhythmias).
Antidepressants: concentration possibly increased
by fluvoxamine; possibly increased paroxetine
concentration; concentration of tricyclics possibly
increased.
Antiepileptics: antagonises anticonvulsant effect.
Antimalarials: avoid with artemether/lumefantrine.
Antivirals: concentration possibly increased by
ritonavir.
Anxiolytics and hypnotics: increased sedative effects. | | Metabolism | Metabolism is by direct glucuronidation by UGT1A4
and oxidative metabolism by cytochrome P450
isoenzymes (predominantly CYP1A2) are the primary
metabolic pathways for asenapine.
Excretion is 50% renal and 50% via the faeces. |
| | Asenapine Preparation Products And Raw materials |
|