- Entacapone
-

- $0.00 / 25kg
-
2024-09-09
- CAS:130929-57-6
- Min. Order: 1kg
- Purity: 98% HPLC
- Supply Ability: 500 kgs
- Entacapone
-

- $40.00 / 1kg
-
2024-08-15
- CAS:130929-57-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Entacapone
-

- $20.00 / 10kg
-
2023-07-31
- CAS:130929-57-6
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 20tons
|
| Product Name: | Entacapone | | Synonyms: | (E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-pmpenamide;Entacapone (150 mg);(2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enaMide;2-PropenaMide,2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-, (2E)-;Entacapone (isomer E);(E)-2-cyano-3-(5-(dihydroxyamino)-3,4-dioxocyclohexa-1,5-dienyl)-N,N-diethylacrylamide;ENTACAPONE;2-cyano-3-(5-dihydroxyamino-3,4-dioxo-1-cyclohexa-1,5-dienyl)-n,n-diethyl-prop-2-enamide | | CAS: | 130929-57-6 | | MF: | C14H15N3O5 | | MW: | 305.29 | | EINECS: | 212-686-0 | | Product Categories: | Histone Methyltransf;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 130929-57-6.mol | 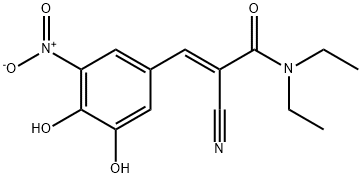 |
| | Entacapone Chemical Properties |
| Melting point | 162-1630C | | Boiling point | 526.6±50.0 °C(Predicted) | | density | 1.392±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO: soluble20mg/mL, clear | | form | powder | | pka | pKa ~4.5(at 25℃) | | color | white to light brown | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. | | InChIKey | JRURYQJSLYLRLN-BJMVGYQFSA-N | | CAS DataBase Reference | 130929-57-6(CAS DataBase Reference) |
| | Entacapone Usage And Synthesis |
| Anti-Parkinson's disease drugs | Entacapone is an anti-Parkinson's disease drug which is successfully developed by Orion Pharma company in Swedish. It is a highly selective potent catechol-O-methyltransferase (COMT) inhibitor, rarely penetrating the blood-brain barrier, and primarily acting in the intestinal tract. It is dose-dependent to decrease levels of 3-OMD in serum and the brain, increasing levodopa, dopamine and DOPAC levels in the brain and significantly reducing the dose which is required to increase dopamine concentration in striatal. Levodopa and carbidopa combining with COMTI can significantly increase the bioavailability of levodopa (3-4 times). Activity of COMTI in red blood cell is reversible. When in 800mg dose, the maximum inhibitory activity is up to 82%, so Entacapone combines with levodopa and carbidopa, which can be used for adjuvant therapy of idiopathic Parkinson's disease. | | Pharmacokinetics | This product is rapid oral absorption, the bioavailability is a dose-dependent with the range of 30% to 45%. In the range of 5~800 mg, pharmacokinetics of Entacapone (abbreviation: Ent) is linear, peak plasma concentration is related to AUC and dose. Food does not affect the absorption of this product, 98% Ent combines with plasma albumin, rarely distributing in tissues. In patients with Parkinson disease (abbreviated: PD), and is required to take levodopa/carbidopa, the peak concentration of Ent arrives within 1~2h. The rate of Ent through the blood-brain barrier is low, the plasma elimination half-life is 1.5~3.5h. After oral administration, Ent (E-configuration) is metabolized to Z-isomer in the blood and is present in plasma and red blood cells. Z-isomer has little impact on the clinical efficacy. Its drug-time curve is similar to Ent. Z-Ent accounts for about 5% of the total AUC. Ent and Z-Ent are acidified by glucose in the liver. After metabolism of Ent: 10% excreted in the urine, 90% of Ent excreted in the feces, only 0.2% excreted in the urine in phony drugs. While taking levodopa in PD patients, and oral Ent 200 mg after elimination half-life of about 1 h, the body has no savings.
Pharmacokinetic study shows that in healthy persons and patients with PD, Ent can increase the bioavailability of levodopa. In the short-term PD patients taking Ent, AUC of levodopa increase 25%, while the long-term taking Ent can increase 50%. AUC of 3-OMD relatively reduces 60%. In these studies, they found that plasma peak time of levodopa will be extended. Single dose of Entacapone (while not taking levodopa/carbidopa), in patients with liver disease, the patient's AUC and Cmax is 2 times of the patients with normal liver function. We should adjust the dosage of the patient. In patients with mild to moderate kidney disease, it is not necessary to adjust the dosage. Kidney patients receiving dialysis can extend dosing interval.
The above information is edited by the Chemicalbook of Liu Yujie. | | Chemical property | Crystals, melting point 162--163 ℃. | | Uses | As COMT inhibitors, it is used to treat Parkinson's disease. | | Production method | 1.83 g 3,4-dihydroxy-5-nitrobenzaldehyde and 1.5g N, N-diethyl-cyanoacetamide and a catalytic amount of piperidine acetate are dissolved in 40ml of dry ethanol , followed by stirring overnight , 2.23 g crude product is obtained, yield 73%, melting point 153~156 ℃.
Heated at 90 ℃, the 3.0 kg crude product is dissolved in 8.0kg acetic acid (or formic acid) containing 80 g HBr (or 40gHCl).It is slowly cooled to 20 ℃, and stirred at this temperature for 20h, then at 15 ℃ stirred for 6h. The precipitated crystals were collected by filtration, carefully washed with cool (4 ℃) 1L toluene-acetic acid (1: l v/v) mixed solution, and washed with 1L cold toluene. It is dried at 45 ℃under vacuum and 2.4kg crystalline pure Entacapone is obtained, yield 80%, melting point 162-163 ℃. | | Description | Entacapone was introduced in Finland, Germany and Sweden as an
adjunctive treatment with L-dopa in Parkinson’s disease. Entacapone is the
second drug in its class to reach the market; it can be obtained by
basecatalyzed condensation of the corresponding benzaldehyde with a
cyanoacetamide. Entacapone is a highly selective and orally-active catechol-0-
methyltransferase (COMT) inhibitor ; by inhibiting metabolism of L-dopa when
given as an adjuvant in patients with Parkinson’s disease, Entacapone markedly
prolongs the effects of L-dopa and improves its bioavailability. Results from
clinical studies showed that 200mglday Entacapone coadministered with L-dopa
lowered the dose of the latter required to reduce fluctuations in motor
performance. | | Chemical Properties | Yellow Crystalline Solid | | Originator | Orion Pharma (Finland) | | Uses | This compound belongs to the cinnamic acid amides. These are amides of cinnamic acids. Used as an adjunct to levodopa / carbidopa in the symptomatic treatment of patients with idiopathic Parkinson's Disease who experience the signs and symptoms of end-of- | | Uses | antiparkinsinian;catechol-O-methyl transferase inhibitor | | Uses | (E)-Isomer of Entacapone polymorphic form A. Peripherally acting inhibitor of catechol-O-methyl transferase (COMT), an enzyme involved in the metabolism of catecholamine neurotransmitters and related drugs. Antiparkinsonian | | Definition | ChEBI: A monocarboxylic acid amide that is N,N-diethylprop-2-enamide in which the hydrogen at position 2 is substituted by a cyano group and the hydrogen at the 3E position is substituted by a 3,4-dihydroxy-5
nitrophenyl group. | | Manufacturing Process | N,N-Diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)acrylamide (2-
Propenamide, 2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl).
A solution containing 1.83 g of 3,4-dihydroxy-5-nitrobenzaldehyde, 1.5 g of
N,N-diethylcyanoacetamide and catalytic amount of piperidine acetate in 40
ml of dry ethanol was refluxed over night. After cooling the solvent was
evaporated in vacuo and the residue was recrystallized from water�dimethylformamide. Yield of desired product was 2.23 g (73%), melting point
153°-156°C. | | Brand name | Comtan(Orion);Comtess. | | Therapeutic Function | Antiparkinsonian | | General Description | Entacapone, (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide (Comtan),is a nitrocatechol that is practically insoluble in water (pKa=4.50). Entacapone is rapidly absorbed after oral administrationand does not cross the BBB. Entacapone does not distributewidely into tissues because of its high plasma proteinbinding and it is completely metabolized before excretion.The main metabolic pathway is by isomerization to the cisisomerfollowed by direct glucuronidation of the parent andthe cis-isomer. The glucuronide conjugates are inactive.Entacapone is eliminated in the feces (90%) and urine (10%).Entacapone is indicated as an adjunct to levodopa/carbidopato treat patients with idiopathic PD who experience the signsand symptoms of end-of-dose wearing off. | | Biochem/physiol Actions | Entacapone is a catechol-O-methyl transferase (COMT) inhibitor. Used in treatment of Parkinson′s disease, entacapone is administered with L-DOPA to inihibit COMT from converting L-DOPA into a compound that cannot cross the blood brain barrier. | | Drug interactions | Potentially hazardous interactions with other drugs Anticoagulants: enhances anticoagulant effect of
warfarinAntidepressants: use with caution in combination
with moclobemide, tricyclics and venlafaxine; avoid
with MAOIs. Dopaminergics: possibly enhances effects of
apomorphine; possibly reduces concentration
of rasagiline; max dose of selegiline is 10 mg in
combination. | | Metabolism | Entacapone undergoes extensive first-pass metabolism to
form glucuronide metabolites.It is eliminated mainly in the faeces with about 10-20%
being excreted in the urine, mainly as glucuronide
conjugates | | storage | Store at RT | | References | 1) Forsberg?et al.?(2003),?Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: a comparative study in the rat; J. Pharmacol. Exp. Ther.,?304?498
2) Merello?et al. (1994),?Effect of entacapone, a peripherally acting catechol-O-methyltransferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson’s disease; J. Neurol. Neurosurg. Psychiatry,?57?186
3) Giovanni?et al. (2010),?Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta amyloid and protect against amyloid-induced toxicity; J. Biol. Chem.,?285?14941
4) Chen?et al. (2016),?Entacapone is an Antioxident More Potent than Vitamin C and Vitamin E for Scavenging of Hypochlorous Acid and Peroxynitrite, and the Inhibition of Oxidative Stress-induced Cell Death; Med. Sci. Monit.,?22?687 |
| | Entacapone Preparation Products And Raw materials |
|