- Ganirelix Acetate
-

- $30.00 / 1box
-
2024-04-28
- CAS:123246-29-7
- Min. Order: 1box
- Purity: 98%
- Supply Ability: 2000kg
- Ganirelix Acetate
-

- $0.00 / 1g
-
2024-04-18
- CAS:123246-29-7
- Min. Order: 50g
- Purity: 99%
- Supply Ability: 100kg
- Ganirelix Acetate
-

- $60.00 / 1kg
-
2024-01-02
- CAS:123246-29-7
- Min. Order: 10kg
- Purity: 0.99
- Supply Ability: 20tons
|
| | Ganirelix Acetate Basic information |
| Product Name: | Ganirelix Acetate | | Synonyms: | Ac-DNal-DCpa-DPal-Ser-Tyr-DHar(Et2)-Leu-Har(Et2)-Pro-DAla -NH2;ganirelix Acetate;GANIRELIX;Ganirelixum;Ganirelix Acetate USP/EP/BP;Ganirelix Impurity 1;Ganirelix Acetate API;3-?bis[[3-?(3 | | CAS: | 123246-29-7 | | MF: | C80H113ClN18O13 | | MW: | 1570.34 | | EINECS: | | | Product Categories: | hormones | | Mol File: | 123246-29-7.mol | 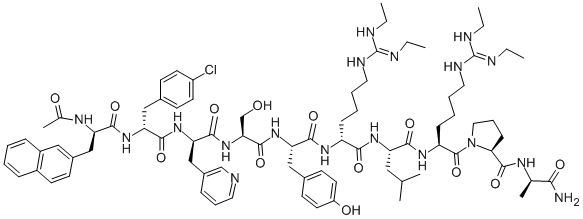 |
| | Ganirelix Acetate Chemical Properties |
| Sequence | Ac-D-Nal-[D-(pCl)Phe]-D-Pal-Ser-Tyr-D-Har(Et2)-Leu-Har(Et2)-Pro-DAla-NH2 |
| | Ganirelix Acetate Usage And Synthesis |
| Originator | Antagon,Organon | | Definition | ChEBI: Ganirelix is a polypeptide. | | Manufacturing Process | The abbreviations for common aminoacids are those recommended by IUPAC�IUB Comission on Biochemical Nomenclature. Other abbreviations useful in
describing the replacements of aminoacids in the natural LH-RH peptide are
following:
Nal(2) - 3-(2-naphthyl)alanyl; p-Cl-Phe - 3-(p-chlorophenyl)alanyl; Pal(3) - 3-
(3-pyridyl)alanyl; ; hArg(Et)2 - NG,NG - bis(ethtyl)homoarginyl; Boc - t�butyloxycarbonyl.
Ganirelix (N-Ac-Nal(2)-D-pCl-Phe-D-Pal(3)-Ser-Tyr-D-hArg(Et)2-Leu-hArg(Et)2-
Pro-Ala-NH2) was prepared using the following side chain protection protocol:
salt protection for L- and D-hArg(Et)2 (as the chloride) and t-butyl protection
for serine.
Amino acids were added to the Nα-Boc-D-Ala-O-Resin (1.0 mmol of resin was
replaced in the reaction vessel of 5.0 L Vega 296 automated solid phase
peptide synthesizer; in the following sequence:
Acetic anhydride
An acetylation (capping of the resin) was done after Ala, Pro and Leu with
N,N'-diisopropyl carbodiimide - 1-hydroxybenztriazole (HBt). Excess HBt (2
equiv.) was used for the coupling of the basic amino acids, hArg(Et)2 and
Pal(3).
The following protocols were used to remove the Nα-protecting group following
each addition.
Program A: The resin was first washed with CH2Cl2 1 times/1 min, TFA-CH2Cl2
(40/60) 1 times/1 min, TFA-CH2Cl2 (40/60) 1 times/30 min, CH2Cl2 2 5
times/1 min, Et3N-CH2Cl2 (5/95) 3 times/1 min, CH2Cl2 4 times/1 min.
Program B: The resin was first washed with CH2Cl2 1 times/1 min, 4-4.5 N
HCl in CH2Cl2/i-PrOH (1/1) 1 times/1 min, 4-4.5 N HCl in CH2Cl2/i-PrOH (1/1)
1 times/30 min, CH2Cl2 3 times/1 min, DMF 1 times/1 min, Et3N-CH2Cl2
(5/95) 3 times/1 min, DMF 1 times/1 min, CH2Cl2 4 times/1 min.
After each deprotecting and washing step, following protocol A or B, the next
amino acid in sequence was added and the resin washed with CH2Cl2 3
times/1 min, MeOH 4 times/1 min, DMF 2 times/1 min and CH2Cl2 4 times/1
min.
Program A was used for the removal of the protecting groups on Ala, Pro, L�hArg(Et)2, Leu and D-Nal(2).
Program B was used for the removal of the protecting groups on D-hArg(Et)2,
Tyr, Ser, D-Pal(3) and p-Cl-Phe.
The crude peptide was first dissolved in 2 M acetic acid and converted to its acetate salt by passage through a column of AG3-X4A resin (Bio-Rad). The
acetate was subjected to chromatography on a silica gel column (CH2Cl2/i�PrOH/MeOH/H2O/HOAc solvent); the acetate fractions dissolved in H2O and
loaded onto a reversed-phase column (Vydec C-18, 15-20 μ), and purified
using acetonitrile/TEAP (pH 3). Fractions of the desired purity were combined
and diluted with water and reloaded on a reversed-phase HPLC column, then
washed with 1% acetic acid in water. The peptide was stripped with a mixture
of MeOH/CH3CN/HOAc/H2O (44/50/1/5). The residue was dissolved in acetic
acid and precipitated over ether, filtered, washed with ether and dried under
vacuum. Amino acid analyses were performed on a Beckman 119CL amino
acid analyzer. Samples for amino acid analyses were hydrolyzed with 6 N HCl
at 110°C for 20 hrs. Analytical HPLC was performed on a Spectra Physics
8800 chromatograph. Synthesis of ganirelix was confirmed by the presence of
a main peak at rt 18 min; no other peak over 1% was noted at rt 16 min. | | Therapeutic Function | LHRH antagonist | | Clinical Use | Ganirelix acetate is an analog of GnRH with substitutions at residues 1, 2, 3, 6, 8, and 10. It is not a superagonist but, rather, is a synthetic decapeptide with high antagonist
activity and the first GnRH antagonist to be marketed. It is approved for the suppression of LH
surges in women who are undergoing ovarian hyperstimulation fertility treatment; LH surges
normally promote ovulation. The goal of this drug is to significantly reduce the number of
medication days necessary to suppress the LH surge, thereby maintaining eggs in the ovaries.
In vitro fertilization (IVF) treatment cycles were historically initiated by the administration of
leuprolide acetate to suppress the premature release of LH. This inhibits ovulation so that the
eggs remain available for retrieval by a fertility specialist. For this purpose, leuprolide acetate usually is injected for as many as 26 days. Clinical studies have shown that ganirelix acetate
can shut down the LH surge in only 5 days of treatment, that the suppression of LH is more
pronounced than that of FSH, and that the shorter treatment time minimizes unpleasant side
effects, such as hot flashes and headaches. |
| | Ganirelix Acetate Preparation Products And Raw materials |
|