| Company Name: |
Tianmen Hengchang Chemical Co., Ltd Gold
|
| Tel: |
027-59322316 15172518801 |
| Email: |
1208480077@qq.com |
| Products Intro: |
Product Name:URANIUM(VI)OXIDE
CAS:1344-58-7
Purity:99 Package:25G
|
|
| | URANIUM(VI) OXIDE Basic information |
| Product Name: | URANIUM(VI) OXIDE | | Synonyms: | URANIUM TRIOXIDE;URANIUM(VI) OXIDE;URANIUM OXIDE YELLOW;UO3;Uranium oxide (UO3);Uranium(VI) oxide, red;triketouranium;trioxouranium | | CAS: | 1344-58-7 | | MF: | O3U | | MW: | 286.03 | | EINECS: | 215-701-9 | | Product Categories: | | | Mol File: | 1344-58-7.mol | 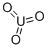 |
| | URANIUM(VI) OXIDE Chemical Properties |
| Melting point | °Cd ec.) | | density | 7,29 g/cm3 | | solubility | insoluble in H2O; soluble in acid solutions | | form | orange-yellow crystals | | color | orange-yellow crystals, crystalline | | Water Solubility | insoluble H2O; soluble acids [MER06] | | EPA Substance Registry System | Uranium trioxide (1344-58-7) |
| RIDADR | 2912 | | HazardClass | 7 | | HS Code | 28441000 |
| | URANIUM(VI) OXIDE Usage And Synthesis |
| Chemical Properties | Uranium trioxide has been isolated in six well-defined stoichiometric modifications as well as a hypostoichiometric modification, UO2. Similar to U3O8, the trioxide decomposes into lower oxides prior to melting or subliming. Even though UO3 is formally U(VI), a small temperature-dependent paramagnetism exists with molar magnetic susceptibility values ranging from 128 to 157×10?6 cm3/mol. A general trend has been observed for the densities of uranium oxides; an inverse proportionality between the O/U ratio and the density of the material. The trioxide does not deviate from this trend with density values ranging from 6.99 to 8.54 g/cm3, depending on the modification. | | Chemical Properties | -100 mesh; has six forms: α is hexagonal brown, β is orange monoclinic, γ is bright yellow rhomb, δ is red cub, ε is brick red tricl, ηis rhomb; UO3 can be obtained by thermal decomposition of uranyl compounds, e.g, carbonates, oxalates nitrates [KIR83] [CER91] | | Uses | Uranium trioxide, UO3, is a versatile solid that has important applications in the nuclear fuel cycle. | | Purification Methods | The oxide is dissolved in HClO4 (to give a uranium content of 5%), and the solution is adjusted to pH 2 by addition of dilute ammonia. Dropwise addition of 30% H2O2, with rapid stirring, precipitated U(VI) peroxide, the pH being held constant during the precipitation, by addition of small amounts of the ammonia solution. Then H2O2 is added until further quantities caused no change in pH. After stirring for 1hour, the slurry is filtered through coarse filter paper in a Büchner funnel, washed with 1% H2O2 acidified to pH 2 with HClO4, then heated at 350o for three days in a large platinum dish [Baes J Phys Chem 60 878 1956]. |
| | URANIUM(VI) OXIDE Preparation Products And Raw materials |
|