- Carboplatin
-

- $120.00 / 1kg
-
2024-04-24
- CAS:41575-94-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Cisplatin
-

- $120.00 / 1kg
-
2024-04-24
- CAS:41575-94-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Carboplatin
-

- $0.00 / 1kg
-
2024-04-15
- CAS:41575-94-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
Related articles - What is Carboplatin?
- Carboplatin is a second-generation platinum compound analog with established activity against a broad spectrum of solid tumors....
- Feb 10,2020
|
| | Carboplatin Chemical Properties |
| Melting point | 228-230°C | | storage temp. | 2-8°C | | solubility | Sparingly soluble in water, very slightly soluble in acetone and in ethanol (96 per cent). | | form | crystal | | color | white | | Water Solubility | Soluble in water. | | Merck | 14,1822 | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChI | InChI=1S/C6H8O4.2H3N.Pt/c7-4(8)6(5(9)10)2-1-3-6;;;/h1-3H2,(H,7,8)(H,9,10);2*1H3;/q;;;+2/p-2 | | InChIKey | OLESAACUTLOWQZ-UHFFFAOYSA-L | | SMILES | C12(CCC1)C(=O)O[Pt]OC2=O.N.N | | EPA Substance Registry System | Carboplatin (41575-94-4) |
| | Carboplatin Usage And Synthesis |
| Description | Carboplatin (Brand name: Paraplatin) is a kind of chemotherapy medication used for the treatment of a series of cancers. It can be used for the treatment of various kinds of cancers including ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma. Moreover, it may also be used for treating some types of testicular cancer. Carboplatin belongs to a kind of alkylating agent. It takes effect through three major mechanisms: (1) Attach the alkyl groups to the DNA bases, further causing DNA fragmentation so that DNA replication is inhibited; (2) Cause DNA damage through inducing the formation of cross-links which prevents DNA from being separated for synthesis or transcription; (3) Induce mispairing of the nucleotides leading to mutations.
| | Uses | Carboplatin is a second-generation platinum compound analog with established activity against a broad spectrum of solid tumors including brain tumors, neuroblastoma, rhabdomyosarcoma, and germ cell tumors. It is commonly used for pediatric cancer and approximately one-third of children with solid tumor are estimated to receive carboplatin at some point during their treatment.
| | Mechanism of action | Once carboplatin penetrates the cell membrane, carboplatin is subjected to hydrolysis becoming positively charged. The hydrolyzed product is capable of reacting with any nucleophile, such as the sulfhydryl groups on proteins and nitrogen donor atoms on nucleic acids. Carboplatin connects to the N7 reactive center on purine bases, which elicits DNA injury that blocks replicative machinery and directs cancer cells towards apoptosis. The spectrum of chemical changes induced by carboplatin within DNA is wide, however, the most prominent is the formation of the 1,2-intrastrand [d(GpG)and d(ApG)] adducts of purines.
| | Description | Carboplatin is a second generation, platinum-containing antineoplastic agent with significantly reduced nephro-, neuro-, and ototoxicity in comparison to cisplatin. It is effective in the treatment of advanced ovarian carcinoma of epithelial origin and small cell carcinoma of the lung. | | Chemical Properties | White Crystals | | Originator | Johnson Matthey (United Kingdom) | | Uses | antitumor agent, | | Uses | Analog of Cisplatin with reduced nephrotoxicity. Antineoplastic | | Uses | Data on carboplatin production have not been found.
Carboplatin is used in chemotherapy to treat cancer, and
more particularly to treat cancer of ovary, embryonal carcinoma
of the testis, microcellular carcinoma of the lung,
neuroblastoma, and squamous cell carcinomas of the head
and neck. | | Indications | Carboplatin (Paraplatin) is an analogue of cisplatin. Its
plasma half-life is 3 to 5 hours, and it has no significant
protein binding. Renal excretion is the major route of
drug elimination.
Despite its lower chemical reactivity, carboplatin
has antitumor activity that is similar to that of cisplatin
against ovarian carcinomas, small cell lung cancers,
and germ cell cancers of the testis. Most tumors that
are resistant to cisplatin are cross-resistant to carboplatin.
The major advantage of carboplatin over cisplatin is
a markedly reduced risk of toxicity to the kidneys, peripheral
nerves, and hearing; additionally, it produces
less nausea and vomiting. It is, however, more myelosuppressive
than cisplatin. Other adverse effects include
anemia, abnormal liver function tests, and occasional allergic
reactions. | | Manufacturing Process | cis-Diammine platinum diiodide was reacted with silver sulfate to give cis-diaquodiammine platinum sulfate. This was reacted with the barium salt of
1,1-cyclobutanedicarboxylic acid to yield Carboplatin. | | Brand name | Paraplatin (Bristol-Myers Squibb). | | Therapeutic Function | Antitumor | | General Description | Carboplatin is available in 50-, 150-, and 450-mg vials for IVadministration in the treatment of ovarian cancer, bladdercancer, germ cell tumors, head and neck cancers, small celllung cancer, and NSCLC. Activation of the agent occurs byaquation in a manner similar to that seen for cisplatin. Thepresence of the chelating 1,1-cyclobutane-dicarboxylateslows this reaction 100-fold and reduces the toxicity of theagent. The sites of alkylation and mechanisms of resistanceare like those seen for cisplatin, and the two agents showcross-resistance. The agent is widely distributed upon IV administration but, because of its greater stability, it bindsslowly to plasma proteins, requiring 24 hours to reach 90%bound drug compared with 4 hours for cisplatin. The agent iseliminated in the urine with a terminal elimination half-lifeof 2 to 6 hours. Adverse effects include myelosuppression,which is dose limiting. Other adverse effects include renaltoxicity, nausea, vomiting, and peripheral neuropathy, butthese occur much less frequently than with cisplatin. | | Pharmaceutical Applications | Carboplatin, cis-diammine(1,1-cyclobutanedicarboxylato)platinum(II), is a second-generation platinum drug.
Its structure is based on cisplatin with the difference that the chloride ligands are exchanged for a bidentate
chelating ligand. A consequence is that carboplatin is less reactive than cisplatin and therefore is less nephrotoxic
and orthotoxic than the parent compound. Unfortunately, it is more myelosuppressive than cisplatin,
which reduces the patients’ white blood cell count and makes them susceptible to infections. Carboplatin
was licensed by the FDA in 1989 under the brand name Paraplatin and has since then gained worldwide
recognition. Carboplatin on its own or in combination with other anticancer agents is used in the treatment
of a variety of cancer types including head and neck, ovarian, small-cell lung, testicular cancer and others.
Carboplatin is a pale-white solid showing good aqueous solubility. The synthesis starts with potassium
tetrachloroplatinate, which is reacted to the orange [PtI4]2- anion. | | Biological Activity | Antitumor agent that forms platinum-DNA adducts. Causes intra- and interstrand DNA crosslinks blocking DNA replication and transcription. Enhances radiation-induced single-strand DNA breakage and displays lower nephrotoxicity than analog cisplatin (cis-Diaminodichloroplatinum ). | | Biochem/physiol Actions | Carboplatin is a platinum-based antineoplastic drug that damages DNA by forming intrastrand cross-links with neighboring guanine residues. Tumors acquire resistance to these drugs through the loss of DNA-mismatch repair (MMR) activity and the resultant decrease in the induction of programmed cell death. | | Mechanism of action | Carboplatin, another square planar Pt(II) complex, forms the same cytotoxic hydrated intermediate as cisplatin but does so at a slower rate, making it a less potent chemotherapeutic agent. | | Clinical Use | This drug induces fewer nonhematological toxicities (e.g., emesis, nephrotoxicity, and ototoxicity) compared to cisplatin, and it is approved for use only in the treatment of ovarian cancer. Unlabeled uses include combination therapy in lung, testicular, and head and neck cancers. | | Side effects | The ultimate damage done to cells as a result of carboplatin use, however, approaches that of cisplatin. The plasma half-life of carboplatin is 3 hours, and the drug is less extensively bound to serum proteins. Excretion is predominantly renal, and doses must be reduced in patients with kidney disease. Suppression of platelets and white blood cells is the most significant toxic reaction of carboplatin use. | | Synthesis | Carboplatin, cis-diamino-(1,1-cyclobutandicarboxylate)platinum(II), is
made from cisplatin by reacting it with a solution of silver nitrate, and then with cyclobu�tan-1,1-dicarboxylic acid to form the desired carboplatin (30.2.5.2). 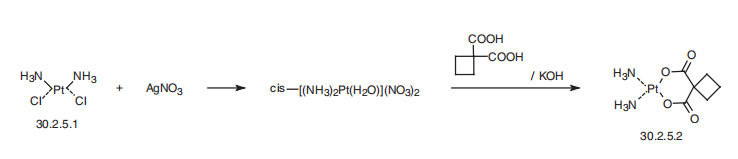
| | Veterinary Drugs and Treatments | Like cisplatin, carboplatin may be useful in a variety of veterinary
neoplastic diseases
including squamous cell carcinomas, ovarian
carcinomas, mediastinal carcinomas, pleural adenocarcinomas,
nasal carcinomas and thyroid adenocarcinomas. Carboplatin’s primary
use currently in small animal medicine is in the adjunctive
treatment (post amputation) of osteogenic sarcomas. Its effectiveness
in treating transitional cell carcinoma of the bladder has been
disappointing; however, carboplatin may have more efficacy against
melanomas than does cisplatin.
Carboplatin, unlike cisplatin, appears to be relatively safe to use
in cats.
Carboplatin may be considered for intralesional use in conditions
such as equine sarcoids or in treating adenocarcinoma in
birds.
Whether carboplatin is more efficacious than cisplatin for certain
cancers does not appear to be decided
at this point, but the
drug does appear to have fewer adverse effects (less renal toxicity
and reduced
vomiting) in dogs. | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: increased risk of nephrotoxicity
and possibly ototoxicity with aminoglycosides,
capreomycin, polymyxins or vancomycin.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis. | | Metabolism | There is little, if any, true metabolism of carboplatin.
Excretion is primarily by glomerular filtration in the
urine, with 70% of the drug excreted within 24 hours,
most of it in the first 6 hours. Approximately 32% of the
dose is excreted unchanged.
Platinum from carboplatin slowly becomes protein
bound, and is subsequently excreted with a terminal half�life of 5 days or more. | | storage | +4°C | | references | [1]. banerji u, sain n, sharp sy, et al. an in vitro and in vivo study of the combination of the heat shock protein inhibitor 17-allylamino-17-demethoxygeldanamycin and carboplatin in human ovarian cancer models. cancer chemother pharmacol, 2008, 62(5): 769-778.
[2]. fiebiger w, olszewski u, ulsperger e, et al. in vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. clin transl oncol, 2011, 13(1): 43-49.
[3]. smith ie, evans bd. carboplatin (jm8) as a single agent and in combination in the treatment of small cell lung cancer. cancer treat rev, 1985, 12 suppl a: 73-75. |
| | Carboplatin Preparation Products And Raw materials |
|