| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Ecothiophate iodide
CAS:513-10-0
Purity:98% Remarks:Ecothiophate iodide is a potent, long-acting cholinesterase inhibitor. It covalently binds by its phosphate group to serine group at the active site of the cholinesterase. It binds irreversibly to cho
|
| Company Name: |
ChemeGen(Shanghai) Biotechnology Co.,Ltd.
|
| Tel: |
18818260767 |
| Email: |
sales@chemegen.com |
| Products Intro: |
Product Name:Echothiophate iodide
CAS:513-10-0
Purity:98% Package:10 mg;50 mg;100 mg;500 mg;1 g;5 g;10 g
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Email: |
marketing@energy-chemical.com |
| Products Intro: |
Product Name:Echothiophate Iodide
CAS:513-10-0
Purity:NULL Package:100mg;1g;2.5g;250mg Remarks:NULL
|
|
| | ECOTHIOPATE IODIDE Basic information |
| Product Name: | ECOTHIOPATE IODIDE | | Synonyms: | ECOTHIOPATE IODIDE;217MI;Ecothiopate;Ecostigmine;ECOTHIOPHATE;ECHOTHIOPHATE;PHOSPHOLINEIODIDE;2-[(Diethoxyphosphinyl)thio]ethanaminium | | CAS: | 513-10-0 | | MF: | C9H23INO3PS | | MW: | 383.227051 | | EINECS: | 208-152-1 | | Product Categories: | | | Mol File: | 513-10-0.mol | 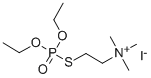 |
| | ECOTHIOPATE IODIDE Chemical Properties |
| RIDADR | 3249 | | HazardClass | 6.1(a) | | PackingGroup | II |
| | ECOTHIOPATE IODIDE Usage And Synthesis |
| Originator | Phospholine Iodide,Ayerst,US,1959 | | Uses | Cholinergic (ophthalmic). | | Uses | Echothiophate is a phosphorylthiocholine with pharmacological action analogous to that
of isofluorophate; however, spontaneous reduction of phosphorylated enzyme occurs
faster after using isofluorophate. It is used in various forms of glaucoma. | | Definition | ChEBI: The iodide salt of ecothiopate. An irreversible acetylcholinesterase inhibitor, it is used an ocular antihypertensive in the treatment of open-angle glaucoma, particularly when other drugs have proved inadequate. | | Manufacturing Process | The reaction is carried out in an atmosphere of nitrogen. To a solution of 4.60
grams sodium (0.20 mol) in 60 cc of methanol is added 14.17 grams β-
dimethylaminoethyl mercaptan hydro chloride (0.10 mol), rinsed in with 10 cc
methanol. Solvent is removed at a water-pump vacuum while blowing with a
slow stream of nitrogen to 100°C/20 mm. To the residue suspended in 150 cc
benzene and cooled in an ice bath is added 17.25 grams
diethylchlorophosphate (0.10 mol) in 3 portions at 10-minute intervals. After
each addition, the temperature increases from about 4° to about 14°C and
then falls. The mixture is stirred in an ice bath for one-half hour and while
warming to room temperature during 2 hours is washed with 35 and 5 cc
portions of water with two 10 cc portions of saturated brine and is dried over
calcium sulfate and filtered.
After removal of solvent by distillation under reduced pressure to 55°C/20
mm, the residue is 23.0 grams crude base (95% theory) as a pale yellow
liquid. A sample of the crude base distills with some decomposition at 105° to
112°C/0.8 mm.
A sample of distilled base in cold isopropanol is treated with excess methyl
iodide, left at room temperature overnight, diluted with 5 volumes of ethyl
acetate and filtered from the methiodide salt. This is purified by crystallization
from mixtures of isopropanol and ethyl acetate, filtering hot to remove an
impurity of low solubility. The pure methiodide is obtained as a white solid, MP
124° to 124.5°C, containing 99 mol percent thiol isomer. | | Brand name | Phospholine Iodide (Wyeth). | | Therapeutic Function | Cholinergic (ophthalmic) | | Clinical Use | Echothiophate iodide has found therapeutic application for the treatment of glaucoma and strabismus. Echothiophate is applied topically as a
solution and is the only irreversible AChEI for the treatment of glaucoma. The decrease in intraocular pressure observed can last up to 4 weeks.
Phosphoester AChEIs exhibit cataractogenic properties; thus, their use should be reserved for patients who are refractory to other forms of
treatment (i.e., short-acting miotics, β-blockers, epinephrine, and possibly, carbonic anhydrase inhibitors). Because of its toxicity, echothiophate
is not used for its systemic action. Selectivity of echothiophate for the AChE catalytic site was enhanced by incorporation in the molecule of a
quaternary ammonium salt functional group two carbons removed from the phosphoryl group. | | Synthesis | Echothiophate, S-(2-trimethylaminoethyl)-O,O-diethylthiophosphate
(13.2.23), is made by reacting diethylchlorophosphoric acid with 2-dimethylaminoethylmer�captane, giving S-(2-dimethylaminoethyl)-O,O-diethylthiophosphate (13.2.22), which is
alkylated by methyliodide, forming echothiophate (13.2.23) [51]. 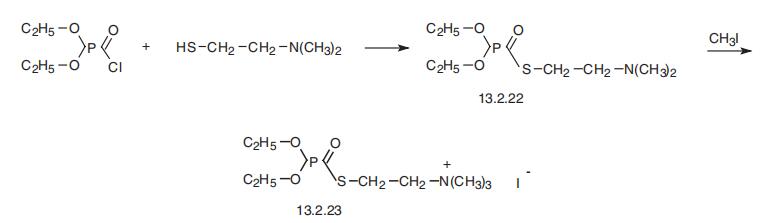
|
| | ECOTHIOPATE IODIDE Preparation Products And Raw materials |
|