- Atorvastatin calcium
-

- $16.00 / 1KG
-
2022-02-25
- CAS:530-78-9
- Min. Order: 1Kg/Bag
- Purity: 99
- Supply Ability: 20tons
- Flufenamic acid
-

- $1.00 / 1g
-
2021-07-20
- CAS:530-78-9
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 50tons
- Flufenamic acid
-

- $1.00 / 1g
-
2021-07-20
- CAS:530-78-9
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 50tons
|
| | Flufenamic acid Basic information |
| | Flufenamic acid Chemical Properties |
| Melting point | 132-135 °C(lit.) | | Boiling point | 373.9±42.0 °C(Predicted) | | density | 1.3380 (estimate) | | vapor pressure | 0Pa at 25℃ | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of flufenamic acid in these solvents is approximately 11, 39, and 59 mg/ml, respectively. | | pka | pKa 3.9 (Uncertain);3.85 (Uncertain) | | form | Crystalline Powder or Chunks | | color | Off-white to gray-green | | Water Solubility | 0.0265 g/L (37 ºC) | | Merck | 14,4132 | | BRN | 1996069 | | LogP | 5.25 | | CAS DataBase Reference | 530-78-9(CAS DataBase Reference) | | NIST Chemistry Reference | Flufenamic acid(530-78-9) |
| Hazard Codes | Xn | | Risk Statements | 22-36/38 | | Safety Statements | 26 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | RTECS | CB4375000 | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 29224999 | | Toxicity | LD50 in mice: 715 mg/kg orally (Zoni) |
| | Flufenamic acid Usage And Synthesis |
| Description | Flufenamic acid (FFA), namely N-(alpha,alpha,alpha-trifluorom-tolyl) anthranilic acid (CI-440), is an aromatic amino acid consisting of anthranilic acid carrying an N-(trifluoromethyl)phenyl substituent. It is an effective drug in the treatment of special types of migraine. Its anti-inflammatory and analgesic effects were recognized in the 1960s (Winder et al., 1963) and thus FFA is included in the family of non-steroidal anti-inflammatory drugs (NSAIDs) with mefenamic, meclofenamic (MFA) and niflumic acids (NA). Anti-inflammatory actions occur mainly through reduction of prostaglandin synthesis from arachidonic acid by inhibiting the cyclo-oxygenases. | | Chemical Properties | Fluomic acid is a Pale-Yellow or light yellow-green crystal or crystalline powder with bitter taste. It is almost insoluble in water and can be dissolved in 50% ethanol. It is a non-hormonal anti-inflammatory and analgesic drug. It was prepared by photolysis of the corresponding benzotriazinone. | | Originator | Flufenamic acid,AroKor Holdings Inc. | | Uses | Flufenamic acid is used for moderate pain and dysmenorrhea, but it should not be used for
more than 1 week due to the possibility of nephrotoxicity, gastrointestinal toxicity, and
anemia. It is frequently used in combination with the anticoagulant warfarin, the effect of
which is strengthened when combined with flufenamic acid. | | Uses | An NSAID found to be a reversible gap junction blocker | | Definition | ChEBI: flufenamic acid is an aromatic amino acid consisting of anthranilic acid carrying an N-(trifluoromethyl)phenyl substituent. An analgesic and anti-inflammatory, it is used in rheumatic disorders. It has a role as an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor, a non-steroidal anti-inflammatory drug, a non-narcotic analgesic and an antipyretic. It is an aromatic amino acid and an organofluorine compound. It derives from a diphenylamine, an anthranilic acid and a (trifluoromethyl)benzene. It is a conjugate acid of a flufenamate. | | Manufacturing Process | A mixture 31.3 g of o-chlorobenzoic acid, 32.2 g of trifluoromethyl-maminobenzene,
3 g of copper powder, 13.8 g of waterless potassium
carbonate and 100 ml amyl alcohol was refluxed for 4 hours. To the cooled
mixture was added 25 ml of 10 N solution NaOH and the mixture was
concentrated and filtrated. Addition to the filtrate hydrochloric acid and water
give a sediment of 2-((3-trifluromethyl)phenyl)aminobenzoic acid. After
recrystallization from hexane 2-((3-trifluromethyl)phenyl)aminobenzoic acid
have melting point 134-136°C. | | Brand name | Arlef (Parke-Davis, Sankyo, Japan), Ansatin (Ono, Japan), Felunamin (Hokuriko, Japan),
Romafen (Biofarma, Turkey). | | Therapeutic Function | Antiinflammatory, Antirheumatic | | Biological Activity | Flufenamic acid is a nonsteroidal anti-inflammatory drug (NSAID). Inhibits calcium-activated chloride channels (CaCCs). Also increases currents through TRPC6 channels and inhibits currents through TRPC3 and TRPC7 channels. | | Mechanism of action | In addition to COX inhibition flufenamate
like other fenamates modifies several ion channel
functions, e.g., inhibition of nonselective
cation conductance, calcium-activated
chloride channels, voltage-gated calcium
channels and potassium channels and
induces blocking of gap junctions. The relevance
of these activities for the analgesic and
anti-inflammatory potential of fenamates is unknown. | | Clinical Use | Flufenamate is a nonsteroidal
anti-inflammatory drug used for the treatment of
mild to moderate pain of musculoskeletal, joint
or soft-tissue origin. It was marketed in a variety
of topical formulations alone or in combination
with other ingredients. | | Safety | Flufenamic acid is used at 600 – 800 mg/d to
provide a beneficial therapeutic effect in chronic
polyarthritis. The adverse effects most often encountered are gastrointestinal disturbances. | | Synthesis | Flufenamic acid, N-(|á,|á,|á-trifluoro-m-tolyl)anthranylic acid (3.2.18),
is synthesized by the reaction of 2-chlorobenzoic acid with 3-trifluoromethylaniline in the
presence of potassium carbonate and copper filings [78,79].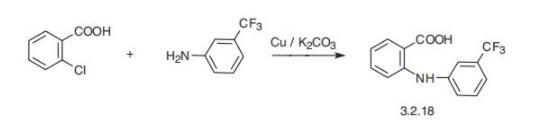 | | storage | Store at RT |
| | Flufenamic acid Preparation Products And Raw materials |
|