- Mivacurium Chloride
-
- $0.00 / 10mg
-
2024-04-11
- CAS:106861-44-3
- Min. Order: 10mg
- Purity: above 98%
- Supply Ability: 10g/month
- Mivacurium Dichloride
-

- $200.00 / 1kg
-
2023-06-26
- CAS:106861-44-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
|
| | Mivacurium chloride Basic information |
| Product Name: | Mivacurium chloride | | Synonyms: | MIVACURIUM CHLORIDE;rac Mivacurium Chloride;rac-BW-B 1090;rac-Mivacron;rel-(1R,1'R)-2,2'-[[(4E)-1,8-Dioxo-4-octene-1,8-diyl]bis(oxy-3,1-propanediyl)]bis[1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-[(3,4,5-trimethoxyphenyl)methyl]- isoquinolinium Chloride;[R-[R*,R*-(E)]]2,2'-(1,8-Dioxo-4-octene-1,8-diyl)bis(oxy-3,1-propanediyl)bis[1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-(3,4,5-trimethoxyphenyl)isoquinolinium] dichloride;rac-BW-B 1090U;-1,2,3,4-Te.trahydro-2-(3-hydroxypropyl)-6,7-dimethoxy-2-methyl-1-(3,4,5-trimethoxy-benzyl)isoquinolinium chloride,(E)-4-octenedioate(2:1) | | CAS: | 106861-44-3 | | MF: | C58H80ClN2O14+ | | MW: | 1064.73 | | EINECS: | 643-006-4 | | Product Categories: | Intermediates & Fine Chemicals;Neurochemicals;Pharmaceuticals;API | | Mol File: | 106861-44-3.mol | 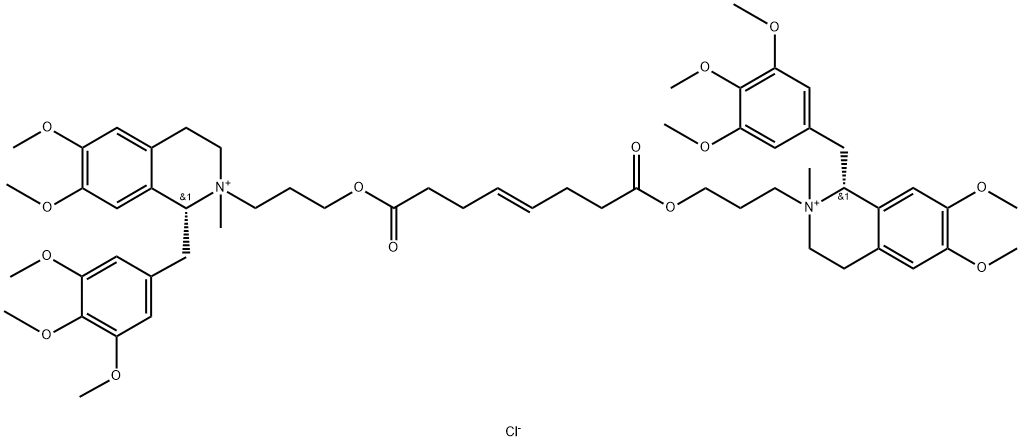 |
| | Mivacurium chloride Chemical Properties |
| alpha | 20D -62.7° (c = 1.9 in water) | | storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere | | solubility | DMSO: Soluble; Water: Soluble | | form | A solid | | Stability: | Hygroscopic | | InChIKey | KFWHZSUNFAPZPW-NVIAPQDINA-M |
| | Mivacurium chloride Usage And Synthesis |
| Description | Mivacurium chloride,a mixture of three stereoisomers, is an intravenously
administered, short-acting skeletal muscle relaxant introduced as an adjunct to general
anesthesia. Structurally mivacurium chloride is closely related to doxacurium chloride
introduced in 1991 by Wellcome as a muscle relaxant. It is a nondepolarizing
neuromuscular blocking agent reportedly with a shorter duration of action and a more
rapid rate of spontaneous recovery than other nondepolarizing agents. In extensive
clinical trials mivacurium chloride was well tolerated with few side effects. | | Description | Mivacurium is an antagonist of nicotinic acetylcholine receptors (nAChRs) and muscarinic M2 and M3 receptors (ED50s = 0.08, 0.3, and 0.1 mg/kg for ex vivo human skeletal muscle nAChRs, guinea pig cardiac M2 receptors, and guinea pig bronchial M3 receptors, respectively). It inhibits acetylcholine-induced activation of neuronal nAChRs (IC50s = 69.04, 3.71, 1.52, and 2.90 for human α3β2-, α3β4-, α4β2-, and α7-containing nAChRs expressed in Xenopus oocytes). Mivacurium also inhibits adult human muscular α1β1εδ-containing nAChRs (IC50 = 3.69 nM in Xenopus oocytes expressing the human recombinant receptor). In vivo, mivacurium inhibits bradycardia and bronchoconstriction induced by vagal stimulation or acetylcholine in guinea pigs. It also induces neuromuscular blockade (ED95 = 80 μg/kg) in sheep with a more rapid onset time than atracurium and vecuronium . Formulations containing mivacurium have been used for pediatric anesthesia. | | Chemical Properties | Off-White Solid | | Originator | Wellcome (United Kingdom) | | Uses | Non-depolarizing neuromuscular blocking agent. Muscle relaxant (skeletal) | | Definition | ChEBI: Mivacurium chloride is a member of isoquinolines. | | Manufacturing Process | To ()-5'-methoxylaudanosine (46.4 g) in methanol (240 mL) was added (-)-
dibenzoyltartaric acid monohydrate (45.2 g). The mixture was heated to
boiling, cooled at 5°C for 16 h and the (S)-(-)-5'-methoxylaudanosinium
dibenzoyltartrate salt (35.6 g, 80%) was filtered and discarded. The mother
liquors were made basic with concentrated aqueous NaOH and evaporated
under vacuum. The solid residue was partitioned between H2O and diethyl
ether. The ether phase was dried and evaporated to an oil (24.9 g). To the oil
in methanol (128 mL) was added (+)-dibenzoyltartaric acid monohydrate
(26.6 g). The mixture was heated to boiling and cooled at 5°C for 16 h.
Crystals were collected and recrystallized from methanol until a constant
specific rotation of [α]D20=+17.7° (1% EtOH) had been achieved. The yield of
(R)-(+)-5'-methoxylaudanosinium dibenzoyltartrate as white crystals was 29.4
g (66%). A portion of the salt (15.0 g) in methanol (200 mL) was made basic
with concentrated aqueous NaOH. The mixture was evaporated under vacuum
and the residue was partitioned between H2O and diethyl ether. The combined
ether layers were dried and evaporated under vacuum to yield 7.2 g (92%) of
(R)-(-)-5'-methoxylaudanosine as an oil.
(R)-(-)-5'-Methoxylaudanosine (7.2 g), 3-chloropropanol (3.5 g), sodium
iodide (5.6 g) and sodium carbonate (0.5 g) were refluxed in 2-butanone (125
mL) for 16 h. The white suspension was filtered hot and solvent removed from
the filtrate under vacuum. The residual gum was trituated with hot ethyl
acetate to remove excess 3-iodopropanol, dissolved in 200 mL methanol and
passed through a column packed with Dowex RTM.1-X8 ion exchange resin
(60 g chloride form). The eluant was stripped of solvent under vacuum to give
N-3-hydroxypropyl-1-(R)-5'-methoxylaudanosinium chloride (8.4 g) as an
amophous solid. The material was assayed by HPLC as a 2.3/1 mixture of the trans/cis diastereomers.
N-3-Hydroxypropyl-1-(R)-5'-methoxylaudanosinium chloride (2.3/1, trans/cis
by HPLC, 2.5 g) was dissolved in 60 mL 1,2-dichloroethane at about 70°C.
(E)-4-Octene-1,8-dioic acid chloride (0.5 g) (K. Sisido, K. Sei, and H. Nozaki,
J. Org. Chem., 1962, 27, 2681) was added and the mixture was stirred at
ambient temperature for 19 h. Solvent was removed under vacuum to give an
amorphous solid which was dissolved in chloroform (25 mL) and washed with
5% aqueous sodium chloride solution to remove unreacted quaternary salts.
The chloroform layer was dried and evaporated under vacuum to give an
amorphous solid. The acid ester impurities were substantially removed by
washing with hot 2-butanone. Residual solvent was evaporated under vacuum
and the resulting amorphous solid was dissolved in methanol, filtered and
lyophilized to give 1.9 g of (E)-(1R,1'R)-2,2'-[4-octenedioylbis
(oxytrimethylene)]bis[1,2,4,3-tetrahydro-6,7-dimethoxy-2-methyl-1-(3,4,5-
trimethoxybenzyl)isoquinolinium] dichloride, which was assayed by HPLC as
44.6% RS-RS (trans-trans) diester, 42.4% RR-RS (cis-trans) diester, 7.5% RR�RR(cis-cis) diester, 4.0% RS (trans) acid ester and 1.5% RR (cis) (E)-
(1R,1'R)-2,2'-[4-Octenedioylbis(oxytrimethylene)]bis[1,2,3,4-tetrahydro-6,7-
dimethoxy-2-methyl-1-(3,4,5-trimethoxybenzyl)isoquinolinium]dichloride acid
ester, [α]D20=-62.7° (1.9% in water). | | Brand name | Mivacron (Abbott). | | Therapeutic Function | Muscle relaxant | | Biological Functions | Mivacurium chloride (Mivacron) is a newer agent
that is chemically related to atracurium. The primary
mechanism of inactivation is hydrolysis by plasma
cholinesterase. Although it is useful for patients with
renal or hepatic disease, some caution is warranted,
since these individuals may have reduced plasma
cholinesterase as a result of the disease.Mivacurium has
an onset of action (1.8 minutes) and duration of effect
(20 minutes) only twice that of succinylcholine, and in
this respect, it is the most similar to succinylcholine of
all of the nondepolarizing agents. | | General Description | Mivacurium chloride, 1,2,3,4-tetrahydro-2-(3-hydroxypropyl)-6,7-dimethoxy-2-methyl-1-(3,4,5-trimethoxybenzyl)isoquinolinium chloride, (E)-4-octandioate (Mivacron), is a mixture of three stereoisomers,the trans-trans, cis-trans, and cis-cis diesters, each ofwhich has neuromuscular blocking properties. The cis-cisisomer is about one tenth as potent as the other isomers.Mivacurium chloride is a short-acting nondepolarizing drugused as an adjunct to anesthesia to relax skeletal muscle.The drug is hydrolyzed by plasma esterases, and it is likelythat anticholinesterase agents used as antidotes could prolongrather than reverse the effects of the drug. |
| | Mivacurium chloride Preparation Products And Raw materials |
|