- Calcium chloride
-

- $100.00 / 50kg
-
2024-09-20
- CAS:10043-52-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton
- Calcium chloride
-

- $6.00 / 1kg
-
2024-09-20
- CAS:10043-52-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
|
| Product Name: | Calcium chloride | | Synonyms: | PELADOW(R) SNOW AND ICE MELT;Calcium chloride,medicinal;Additive Screening Solution 21/Fluka kit no 78374, Calcium chloride solution;calcium chloride anhydrus for technical;calcium chloride anhydrous for food;CACL2 (CALCIUM CHLORIDE);CalciuM chloride, 96%, for biocheMistry, anhydrous;CalciuM chloride, 96%, for analysis, granules | | CAS: | 10043-52-4 | | MF: | CaCl2 | | MW: | 110.98 | | EINECS: | 233-140-8 | | Product Categories: | Inorganic Salt, Food Additives;Crystal Grade Inorganics;20: Ca;Beaded Materials;Metal and Ceramic Science;ACS Grade;Calcium;Chemical Synthesis;Essential Chemicals;Inorganic Salts;Materials Science;Research Essentials;Solutions and Reagents;Synthetic Reagents;A - FMolecular Biology;BioUltraProtein Structural Analysis;DNA&RNA Purification;Molecular Biology Reagents;Optimization ReagentsMolecular Biology;Reagents;X-Ray Crystallography;Calcium SaltsEssential Chemicals;Technical Grade;Adsorbents, Filter Aids and Drying Agents;Metal and Ceramic Science;Other Drying AgentsEssential Chemicals;Routine Reagents;A - F;Salt Solutions;Volumetric Solutions;CalciumSerum-free Media;Companion Products and Reagents;Insect Platform;Calcium Salts;CalciumMetal and Ceramic Science;Crystal Grade Inorganics;Inorganic Salts;Salts;Synthetic Reagents;CA - CGSpectroscopy;AAS;AAS CRMsAlphabetic;AASSpectroscopy;Application CRMs;C;Matrix Selection;Nitrate;Spectroscopy;Pyridines;Pharmaceutical intermediates;ACS GradeSynthetic Reagents;Essential Chemicals;Inorganics;chloride;INORGANIC & ORGANIC CHEMICALS;10043-52-4 | | Mol File: | 10043-52-4.mol |  |
| | Calcium chloride Chemical Properties |
| Melting point | 772 °C (lit.) | | Boiling point | 1935 °C/1 atm (lit.) | | density | 1.086 g/mL at 20 °C | | vapor pressure | 0.01 mm Hg ( 20 °C) | | refractive index | n20/D 1.358 | | Fp | >1600°C | | storage temp. | Store at +5°C to +30°C. | | solubility | H2O: soluble | | form | powder | | color | White to gray | | Specific Gravity | 2.15 | | PH | 8-10 (100g/l, H2O, 20℃) | | Water Solubility | 740 g/L (20 ºC) | | Sensitive | Hygroscopic | | λmax | λ: 260 nm Amax: 0.04
λ: 280 nm Amax: 0.02 | | Crystal Structure | CaCl2 type | | crystal system | Nogata | | Merck | 14,1659 | | Space group | Pnnm | | Lattice constant | | a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.624 | 0.643 | 0.42 | 90 | 90 | 90 | 0.1685 |
| | Stability: | Stable. Incompatible with zinc, water, strong acids, methyl vinyl ether, bromine trifluoride, boron oxide, calcium oxide. Hygroscopic. | | InChIKey | UXVMQQNJUSDDNG-UHFFFAOYSA-L | | CAS DataBase Reference | 10043-52-4(CAS DataBase Reference) | | NIST Chemistry Reference | Calcium dichloride(10043-52-4) | | EPA Substance Registry System | Calcium chloride (10043-52-4) |
| | Calcium chloride Usage And Synthesis |
| Chemical Properties | Calcium chloride, CaC12, is colorless deliquescent solid that is soluble in water and ethanol. It is formed from the reaction of calcium carbonate and hydrochloric acid or calcium hydroxide and ammonium chloride. It is used in medicine, as an antifreeze, and as a coagulant.

Calcium Chloride Powder
| | Uses | Calcium chloride (CaCl2) has many uses. It is used as a drying agent and to melt ice and snow on highways, to control dust, to thaw building materials (sand, gravel, concrete, and so on). It is also used in various food and pharmaceutical industries and as a fungicide.
| | Description | Tumorigen,Mutagen, Human Data; Hormone. Calcium chloride is usedas road salt for melting snow, a drying agent in desiccators,for dehydrating organic liquids and gases, in refrigerationbrines and antifreeze, as a dust-proofing agent, food additive, concrete hardening accelerator, and others.Incompatibilities: The solution in water is a weak base.Reacts with zinc in presence of moisture, forming highly526 Calcium chlorideflammable hydrogen gas. Dissolves violently in waterwith generation of much heat. Incompatible with water,bromine trifluoride; 2-furan, percarboxylic acid. Mayattack some building materials and metals in the presenceof moisture. | | Chemical Properties | Calcium chloride, CaC12, is colorless deliquescent solid that is soluble in water and ethanol. It is formed from the reaction of calcium carbonate and hydrochloric acid or calcium hydroxide and ammonium chloride. It is used in medicine, as an antifreeze, and as a coagulant. | | Chemical Properties | Calcium chloride occurs as a white or colorless crystalline powder,
granules, or crystalline mass, and is hygroscopic (deliquescent). | | Physical properties | White crystal, powder or flake; highly hygroscopic; the compound and its solutions absorb moisture from the air at various rates depending on calcium chloride concentrations, relative humidity and vapor pressure of water in the air, temperature, surface area of exposed material, and the rate of air circulation; at 40% and 95% relative humidity and 25°C, one gram anhydrous calcium chloride may absorb about 1.4 g and 17 g water, respectively. (Shearer, W. L. 1978 . In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., vol. 4, pp. 432-6. New York: Wiley Interscience); density 2.15, 2.24, 1.85, 1.83 and 1.71 g/cm3 for the anhydrous salt and its mono-, di-, tetra- and hexahydrates, respectively; anhydrous salts melts at 772°C, while the mono-, di-, tetra- and hexahydrates decompose at 260°, 175°, 45.5° and 30°C, respectively; the anhydrous salt vaporizes at 1,935°C; highly soluble in water, moderate to high solubility in alcohol.
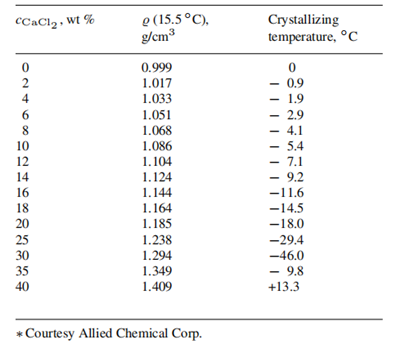
Densities and crystallizing temperatures of commercial calcium chloride solutions (Courtesy Allied Corp.):
 | | Occurrence | Calcium chloride may be found in nature as the mineral tachhydrite, CaCl2?2MgCl2?12H2O. It also is found in other minerals. Its concentration in sea water is about 0.15%.
Calcium chloride has several industrial applications. The major applications of this compound are in deicing of roads, dust control, imparting stability to roads and buildings, and to improve traction in tractor tires. It is mixed with ice to make freezing mixtures. Hexahydrate mixed with crushed ice can lower the temperature of the cooling bath to below -50°C. It also is used as a desiccant for dehydrating gases and liquids. It is added to cement in various proportions to manufacture different types of concrete. Other uses are in adhesives, to lower gel temperatures, and as a calcium source in liquid feed supplements for dairy cattle. Also, the compound is used to control particle size development and reduce coalescence in plastics. | | Uses | Obtained as a by-product in the manufacture of potassium
chlorate. The white crystals, soluble in water and alcohol,
are deliquescent and must be kept in a well-stoppered bottle.
Calcium chloride was used in iodized collodion formulas and
in collodion emulsions. It was also an important desiccating
substance used in tin calcium tubes designed to store presensitized
platinum papers. | | Uses | Calcium Chloride is a general purpose food additive, the anhydrous
form being readily soluble in water with a solubility of 59 g in 100 ml
of water at 0°c. it dissolves with the liberation of heat. it also exists
as calcium chloride dihydrate, being very soluble in water with a
solubility of 97 g in 100 ml at 0°c. it is used as a firming agent for
canned tomatoes, potatoes, and apple slices. in evaporated milk, it
is used at levels not more than 0.1% to adjust the salt balance so as
to prevent coagulation of milk during sterilization. it is used with
disodium edta to protect the flavor in pickles and as a source of
calcium ions for reaction with alginates to form gels. | | Uses | calcium chloride is an astringent. It also helps improve the reaction among certain ingredients used in cosmetic formulations. This inorganic salt is no longer commonly used in skin care products and is being replaced with potassium chloride. | | Uses | Calcium chloride is one of the most versatile of the
basic chemicals.It has several common applications such as brine for
refrigeration plants, ice and dust control on roads, and
in concrete. The anhydrous salt is also widely used as
a desiccant, where it will absorb so much water that it
will eventually dissolve in its own crystal lattice water
(water of hydration). It can be produced directly from
limestone, but large amounts are also produced as
a by-product of the “Solvay Process” (which is a process
to produce soda ash from brine).
Calcium chloride is also commonly used as an additive
in swimming pool water as it increases the “calcium
hardness” value for the water.Other industrial applications include use as an additive
in plastics, as a drainage aid for wastewater treatment,
as an additive in fire extinguishers, as an
additive in control scaffolding in blast furnaces, and as
a thinner in “fabric softeners”.
Calcium chloride is commonly used as an “electrolyte”
and has an extremely salty taste, as found in sports
drinks and other beverages such as Nestle bottled water.
It can also be used as a preservative to maintain firmness
in canned vegetables or in higher concentrations in
pickles to give a salty taste while not increasing the
food’s sodium content. It is even found in snack foods,
including Cadbury chocolate bars.In brewing beer, calcium chloride is sometimes used
to correct mineral deficiencies in the brewing water. It
affects flavor and chemical reactions during the brewing
process, and it can also affect yeast function during
fermentation.
Calcium chloride can be injected as intravenous
therapy for the treatment of “hypocalcemia” (low serum
calcium). It can be used for insect bites or stings (such as
Black Widow spider bites), sensitivity reactions,
particularly when characterized by “urticaria” (hives). | | Uses | For the treatment of hypocalcemia in those conditions requiring a prompt increase in blood plasma calcium levels, for the treatment of magnesium intoxication due to overdosage of magnesium sulfate, and used to combat the deleterious effects of hyperkalemi | | Uses | Calcium chloride is highly hygroscopic and is often used as a desiccant. | | Production Methods | Calcium chloride is a principal byproduct from the Solvay process. | | Preparation | Calcium chloride is obtained as a by-product in the manufacture of sodium carbonate (soda ash) by ammonia-soda (Solvay) process. The process involves the reaction of sodium chloride with calcium carbonate and ammonia. Calcium chloride is currently produced in bulk amounts by evaporation of natural underground brines. In the laboratory, calcium chloride can be prepared by treating limestone with hydrochloric acid followed by evaporation of solution to obtain crystals. The crystals are dehydrated to obtain anhydrous salt. Calcium oxide or hydroxide may be used instead of carbonate. | | Definition | calcium chloride: A white deliquescentcompound, CaCl2, which issoluble in water; r.d. 2.15; m.p.782°C; b.p. >1600°C. There are anumber of hydrated forms, includingthe monohydrate, CaCl2.H2O, the dihydrate,CaCl2.2H2O (r.d. 0.84), andthe hexahydrate, CaCl2.6H2O (trigonal;r.d. 1.71; the hexahydrate loses4H2O at 30°C and the remaining2H2O at 200°C). Large quantities of itare formed as a byproduct of theSolvay process and it can be preparedby dissolving calcium carbonateor calcium oxide in hydrochloricacid. Crystals of the anhydrous saltcan only be obtained if the hydratedsalt is heated in a stream of hydrogenchloride. Solid calcium chloride isused in mines and on roads to reducedust problems, whilst the molten saltis the electrolyte in the extraction ofcalcium. An aqueous solution of calciumchloride is used in refrigerationplants. | | Brand name | Cal Plus (Mallinckrodt). | | General Description | Calcium Chloride (CaCl2) is a water soluble ionic crystal with a high enthalpy change of solution. It is majorly derived from limestone and is a by-product of the Solvay process. It is an anhydrous salt that has a hygroscopic nature and can be used as a desiccant. | | Air & Water Reactions | Deliquescent. Water soluble. Adding Calcium chloride to hot water caused violent boiling, [MCA Case History No. 69]. | | Reactivity Profile | Bromine trifluoride rapidly attacks the following salts: barium chloride, cadmium chloride, Calcium chloride, cesium chloride, lithium chloride, silver chloride, rubidium chloride, potassium bromide, potassium chloride, potassium iodide, rhodium tetrabromide, sodium bromide, sodium chloride, and sodium iodide [Mellor 2 Supp. 1:164, 165 1956]. Long term exposure of Calcium chloride solution upon a zinc coated galvanized iron vessel caused slow evolution of hydrogen which ignited and exploded [Bretherick, 5th Ed., 1995]. | | Health Hazard | Inhalation causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Contact with eyes (particularly by dust) causes irritation and possible transient corneal injury. Contact of solid with dry skin causes mild irritation; strong solutions can cause marked irritation, even a superficial burn. | | Flammability and Explosibility | Non flammable | | Pharmaceutical Applications | The main applications of calcium chloride as an excipient relate to
its dehydrating properties and, therefore, it has been used as an
antimicrobial preservative, as a desiccant, and as an astringent in
eye lotions.
Therapeutically, calcium chloride injection 10% (as the dihydrate
form) is used to treat hypocalcemia. | | Safety Profile | Moderately toxic by
ingestion. Poison by intravenous,
intramuscular, intraperitoneal, and
subcutaneous routes. Human systemic
effects: dermatitis, changes in calcium.
Questionable carcinogen with experimental
tumorigenic data. Mutation data reported.
Reacts violently with (B203 + CaO), BrF3.
Reaction with zinc releases explosive
hydrogen gas. Catalyzes exothermic
polymerization of methyl vinyl ether.
Exothermic reaction with water. When
heated to decomposition it emits toxic
fumes of Cl-. See also CALCIUM
COMPOUNDS and CHLORIDES. | | Safety | Calcium chloride is used in topical, ophthalmic, and injection
preparations. The pure form of calcium chloride is toxic by
intravenous, intramuscular, intraperitoneal, and subcutaneous
routes, and moderately toxic by ingestion, causing stomach and
heart disturbances. It is a severe eye irritant and can cause
dermatitis.
LD50 (mouse, IP): 0.21 g/kg
LD50 (mouse, IV): 0.042 g/kg
LD50 (mouse, oral): 1.94 g/kg
LD50 (mouse, SC): 0.82 g/kg
LD50 (rat, IM): 0.025 g/kg
LD50 (rat, IP): 0.26 g/kg
LD50 (rat, oral): 1.0 g/kg
LD50 (rat, SC): 2.63 g/kg | | Potential Exposure | Calcium chloride is used as road salt
for melting snow, a drying agent in desiccators, for dehydrating organic liquids and gases, in refrigeration brines
and antifreeze, as a dust-proofing agent, food additives,
concrete hardening accelerator, and others. May react with
strong oxidizers. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | storage | Calcium chloride is chemically stable; however, it should be
protected from moisture. Store in airtight containers in a cool, dry
place. | | Shipping | There are no label or maximum shipping quantity requirements set by DOT. | | Properties and Applications |
|
ITEMS
|
SPECIFICATION
|
|
APPEARANCE
|
WHITE,HARD ODORLESS FLAKE,
POWDER,PELLET,GRANULE
|
|
CALCIUM CHLORIDE(As CaCl2)
|
94% min
|
|
MAGNESIUM&ALKALI METAL SALT (As NaCl)
|
3.5% max
|
|
WATER INSOLUBLE MATTER
|
0.2% max
|
|
ALKALINITY(As Ca(OH)2)
|
0.20% max
|
|
SULFATE (As CaSO4)
|
0.20% max
|
|
pH VALUE
|
7-11
|
|
As
|
5 ppm max
|
|
Pb
|
10 ppm max
|
|
Fe
|
10 ppm max
|
| | Purification Methods | It is available as fused granules or cubic crystals. It is very hygroscopic, very soluble in H2O (exothermic), and EtOH. Store it in a tightly closed container. [Ehrlich in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 931 1963.] | | Incompatibilities | Calcium chloride is incompatible with soluble carbonates, phosphates,
sulfates, and tartrates. It reacts violently with bromine
trifluoride, and a reaction with zinc releases explosive hydrogen gas.
It has an exothermic reaction with water, and when heated to
decomposition it emits toxic fumes of chlorine. | | Regulatory Status | GRAS listed. Included in the FDA Inactive Ingredients Database
(injections, ophthalmic preparations, suspensions, creams).
Included in medicines licensed in the UK (eye drops; intraocular
irrigation; vaccines; injection powders for reconstitution; nebulizer
solution; oral suspension). |
| | Calcium chloride Preparation Products And Raw materials |
| Raw materials | Hydrochloric acid-->Calcium carbonate-->Calcium hydroxide-->Calcium oxide-->Sodium chlorate-->CALCIUM CARBONATE-->Calcium chloride hexahydrate-->Calcium chloride solution 36-40%, (1box=27kgs)-->Calcium chloride dihydrate | | Preparation Products | 1-METHYL-3-PHENYL-1H-PYRAZOLE-5-CARBALDEHYDE-->4-CHLORO-2-METHYLBENZALDEHYDE-->(R)-1-Boc-3-(hyroxymethyl)piperidine-->Pentoxyverine-->3-AMIDINOPYRIDINIUM CHLORIDE-->3-Methylbutyl 3-methylbutanoate-->1,3-Dimethoxybenzene-->(1,1-DIMETHYL-PROP-2-YNYL)-HYDRAZINE-->3-(2-THIENYL)PROPIONIC ACID-->(S)-3-(Boc-amino)pyrrolidine-->1-[4-(BROMOMETHYL)PHENYL]-1H-PYRAZOLE-->N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride-->Allyl hexanoate-->Ethyl Hexanoate-->Pigment Red 57:1-->1-(2-ETHOXYETHYL)PIPERAZINE-->Pigment Red 48:2-->1-Bromooctadecane-->Isoamyl acetate-->HEPARIN CALCIUM-->Butyl butyrate-->polymer bactericidal flocculent-->N-Benzylcinchonidinium chloride-->3,5-DIMETHYL-4-NITROISOXAZOLE-->Ethyl oleate-->5-Methyl-4-nitroisoxazole-->Calcium-->N-Allylthiourea-->materials of oral delivery system for peptide drugs and their controlled release-->Isoamyl butyrate-->Dye-fixing agent G-->4-CHLOROPHENETOLE-->Butanethiol-->2-Fluoro-p-Xylene-->Phenyl isothiocyanate-->1,2-Dibromopropane-->2,3-DIMETHYL-1,3-BUTADIENE-->Cinnamyl chloride-->2-Bromoethyl ethyl ether-->1-ETHOXYNAPHTHALENE |
|