| Product Name: | CHLORINE TRIFLUORIDE | | Synonyms: | Chlorine fluoride;Chlorine fluoride (ClF3);chlorinefluoride;chlorinefluoride(cl2f6);chlorinefluoride(clf3);chlorinetrifluoride(clf3);Chlorotrifluoride;ClF3 | | CAS: | 7790-91-2 | | MF: | ClF3 | | MW: | 92.45 | | EINECS: | 232-230-4 | | Product Categories: | | | Mol File: | 7790-91-2.mol | 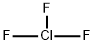 |
| | CHLORINE TRIFLUORIDE Chemical Properties |
| Melting point | -83°C | | Boiling point | 11,75°C | | density | 1,8 g/cm3 | | solubility | reacts with H2O | | form | gas | | Water Solubility | violently hydrolyzed by H2O [MER06] | | Exposure limits | Ceiling 0.1 ppm (~0.4 mg/m3)(ACGIH,
MSHA, NIOSH, and OSHA); IDLH 20 ppm
(NIOSH). | | Stability: | Strong oxidizer. Incompatible with, and may ignite or react violently with, combustible materials, including most organic compounds. Decomposes in water. Incompatible with moisture. | | CAS DataBase Reference | 7790-91-2(CAS DataBase Reference) | | EPA Substance Registry System | Chlorine trifluoride (7790-91-2) |
| | CHLORINE TRIFLUORIDE Usage And Synthesis |
| Preparation | Chlorine trifluoride is obtained by heating chlorine or chlorine monofluoride with fluorine:
Cl2 + 3F2 2ClF3
ClF + F2 ClF3
The gas is purified by distillation in a special steel apparatus.
| | Reference | [1]Handbook-of-inorganic-chemicals
| | Description | Chlorine trifluoride is a greenish yellow,almost colorless, liquid (below 12℃/53°F) or colorless gaswith a sweet, irritating odor. Shipped as a liquefied compressed gas. Molecular weight=92.45; Boilingpoint=11.8℃; Freezing/Melting point=2 76.3℃; Vaporpressure=1.4 atm; Relative vapor density (air=1)=3.21.Hazard Identification (based on NFPA 704 M RatingSystem): Health 4, Flammability 0, Reactivity 3 (Oxidizer).Reacts with water. | | Chemical Properties | yellowish gas or liquid | | Chemical Properties | Chlorine trifluoride is a greenish yellow,
almost colorless, liquid (below 12C/53F), or colorless gas
with a sweet, irritating odor. Shipped as a liquefied compressed gas. | | Physical properties | Colorless gas; sweetish but suffocating odor; density of the liquid 1.77 g/mL at 13°C; condenses to a greenish yellow liquid at 11.75°C; freezes to a white solid at -76.3°C; reacts violently with water. | | Uses | Chlorine trifluoride is used as a fluorinatingagent, as a rocket propellant, in processingof nuclear reactor fuel, and in incendiaries.It is also used as an inhibitor of pyrolysis offluorocarbon polymers. | | Uses | Fluorinating agent; incendiary; igniter
and propellant for rockets; in nuclear reactor
fuel processing; pyrolysis inhibitor for fluorocarbon
polymers | | Uses | Fluorinating agent; in nuclear reactor fuel processing; incendiary; igniter and propellant for rockets; pyrolysis inhibitor for fluorocarbon polymers. | | Definition | ChEBI: Trifluorochlorine is a halohalide. | | General Description | A colorless gas or green liquid with a pungent odor. Boils at 53°F. CHLORINE TRIFLUORIDE reacts with water to form chlorine and hydrofluoric acid with release of heat. Contact with organic materials may result in spontaneous ignition. CHLORINE TRIFLUORIDE is corrosive to metals and tissue. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Under prolonged exposure to fire or intense heat the container may violently rupture and rocket. | | Air & Water Reactions | A violent reaction occurs with water or ice generating acidic HF and chlorine, [Sidgwick, 1156(1950)]. The release of CHLORINE TRIFLUORIDE to the atmosphere rapidly generates two toxic reaction products: HF and Chlorine Dioxide, [Lombardi, D.A. and M.D. Cheng 1996. "Modeling Accidental Releases of CHLORINE TRIFLUORIDE to the Atmosphere," Paper No. 96-WP66B.02, presented at the 89th Annual Meeting of the Air and Waste Management Association, Nashville, Tennessee, June 23-26]. | | Reactivity Profile | CHLORINE TRIFLUORIDE is a low-boiling liquid (b.p. 12° C), in gaseous state irritating and toxic. A highly reactive oxidant reagent, spontaneously flammable, used as a rocket propellant. Incompatible with fuels, nitro compounds. Interaction with water is violent and may be explosive, even with ice [Sidgwick, 1950, p. 1156]. Immediate explosive reaction with hydrocarbons or halocarbons even at -70° C [Brower, K. R., J. Fluorine Chem., 1986, 31, p. 333]. Solution with carbon tetrachloride capable of detonation, solutions with nitroaryl compounds (TNT, hexanitrobiphenyl) or highly chlorinated compounds are extremely shock-sensitive. Violent, sometimes explosive reaction with hydrogen containing materials, e.g., acetic acid, ammonia, benzene, ether, coal gas, hydrogen, hydrogen sulfide, methane, or fluoroamino compounds. Ignition with fibrous materials (cotton, paper, wood). [Mellor, 1956, vol. 2, suppl. 1, p. 155]. Explosive gaseous products (chlorodifluoroamine) formed with ammonium fluoride or ammonium hydrogen fluoride [Gardner, D. M. et al., Inorg., Chem., 1963, 2, p. 413]. Ignition on contact with iodine, boron-containing materials (boron powder, tetraboron carbide, boron-aluminum), fibrous or finely divided refractory materials (asbestos, glass, wool, sand, tungsten carbide). Violent reaction with mineral acids (nitric acid, sulfuric acid), chromium trioxide, ruthenium metal, selenium tetrafluoride. [Bretherick, 5th ed., 1995, p. 1235]. CHLORINE TRIFLUORIDE is a hypergolic oxidizer and contact with a number of metals and their oxides (aluminum, antimony, arsenic, calcium, copper, iridium, iron, lithium, lead, magnesium, molybdenum, osmium, potassium, rhodium, sodium, selenium, silver, tellurium, tin, tungsten, zinc), nonmetals (phosphorus, silicon, sulfur), salts (mercury iodide, potassium iodide, silver, nitrate, potassium carbonate) will result in a violent reaction often followed by ignition [Mellor, 1956, vol. 2, suppl. 1, p. 155; Sidgwick, 1950, p. 1156]. | | Hazard | Explodes in contact with organic materials or with water. Dangerous fire risk. A poison,
very toxic, corrosive to skin. Lung damage, eye,
and upper respiratory tract irritant. Questionable
carcinogen. | | Health Hazard | Chlorine trifluoride is a severe irritant tothe skin, eyes, and mucous membranes.Exposure to this gas can cause lung dam age. A 30-minute exposure to 400 ppm waslethal to rats. It decomposes in the presenceof moisture to chlorine, chlorine dioxide,and hydrogen fluoride, all of which arehighly toxic. Chronic inhalation study on ani mals for a period of 6 months (6 hours/day,5 days/week) indicated that at an exposurelevel of nearly 1 ppm the early symptomswere sneezing, salivation, and expulsionof frothy fluid from the mouth and nose(ACGIH 1986). This progressed to mus cle weakness, pneumonia, and lung damage.Some animals died.
In humans, exposure to this gas can pro duce severe injury to the eyes, skin, andrespiratory tract, and pulmonary edema. Theliquid is severely corrosive to the skin andeyes. Skin contact can cause painful burns. | | Health Hazard | Inhalation causes extreme irritation of respiratory tract; pulmonary edema may result. Vapors are very irritating to eyes and skin; liquid causes severe burns. | | Fire Hazard | Nonflammable gas; dangerously reactive.
Chlorine trifluoride reacts explosively with
water, forming hydrogen fluoride and chlo rine. It reacts violently with most elements
and common substances. Paper, cloth, wood,
glass, wool, charcoal, and graphite burst
into flame in contact with the liquid. The
vapors, even when diluted, can set fire to
organic compounds. Reactions with most metals are vigorous to violent, often caus ing a fire. It catches fire when mixed with
phosphorus, arsenic, antimony, silicon, sul fur, selenium, tellurium, tungsten, osmium,
and rhodium (Mellor 1946, Suppl. 1956).
Among the alkali- and alkaline–earth metals,
reaction is violent with potassium at ordinary
temperatures, and with sodium, calcium, or
magnesium it reacts violently at elevated
temperatures. Violent reaction occurs with
oxides, sulfides, halides, and carbides of
metals, causing flames. Chlorine trifluoride
attacks sand, glass, and asbestos. Prolonged
contact can ignite glass. Explosive reactions
occur with many common gases, includ ing hydrogen, lower hydrocarbons, carbon
monoxide, ammonia, hydrogen sulfide, and
sulfur dioxide. Reactions with mineral acids
and alkalies are violent.
In case of a small fire involving chlorine
trifluoride, use a dry chemical or water
spray in large amounts (NFPA 1997). Allow
large fires to burn. Avoid contact of chlorine
trifluoride with the body or with protective
clothing. | | Safety Profile | Human poison by
inhalation. An eye irritant. See also
FLUORIDES, CHLORINE, and
FLUORINE. Spontaneously flammable. A
powerful oxidant whch may react violently
with oxidzable materials. A rocket
propellant.
Explosive reaction with water,
bis (trifluoromethyl) sulfide or -disulfide,
polychlorotrifluoroethylene,
trifluoromethanesulfenyl chloride, and other
hydrogencontaining materials (e.g.,
ammonia, coal gas, hydrogen, hydrogen
sulfide, methane, acetic acid, benzene, ether,
cotton, paper, wood). Forms shock-sensitive
explosive mixtures with highly chlorinated
compounds (e.g., carbon tetrachloride),
nitroaryl compounds (e.g., trinitrotoluene,
hexanitrobiphenyl, hexanitrodiphenyl amine,
hexanitrodiphenyl sulfide, hexanitrobphenyl
ether). Reaction with ammonium fluoride or
ammonium hydrogen fluoride forms
explosive gaseous products.
materials, iodine, finely dvided refractory
materials (e.g., asbestos, glass wool, sand,
tungsten carbide), fluorinated polymers
(with flowing trifluoride).
sulfuric), chromium trioxide, ruthenium,
selenium tetrafluoride (above 106℃),
metals, metal oxides, metal salts, nonmetals,
nonmetal salts, organic matter, glass wool,
acetic acid, Al, Sb, As, Cu, Ir, Fe, Pb, Mg,
Mo, Os, P, Ir, Rh, Se, Si, Ag, Na, S, Te, Sn,
W, Zn, oxides, CO, graphite, HgI2, HNO3,
Ignition on contact with boron-containing
Violent reaction with acids (e.g., nitric or K2CO3, KI, rubber, AgN3, AgNO3, NaOH,
V2P5, wo3. Incompatible with fuels, nitro
compounds. When heated to decomposition
or in reaction with water or steam it emits
toxic fumes of Fand Cl-. | | Potential Exposure | Chlorine trifluoride is used as a fluorinating agent. It may be used as an igniter and propellant in
rockets. It is used in nuclear fuel processing. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med?ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediately620 Chlorine trifluoridewith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray. If frostbite hasoccurred, seek medical attention immediately; do NOT rubthe affected areas or flush them with water. In order to prevent further tissue damage, do NOT attempt to remove frozen clothing from frostbitten areas. If frostbite has NOToccurred, immediately and thoroughly wash contaminatedskin with soap and water. | | storage | Chlorine trifluoride is stored and shippedin special steel cylinders. It is stored inmoisture-free, cool, and isolated areas sepa rated from other chemicals. The cylinders arekept upright, covered, and protected againstphysical damage. | | Shipping | UN1749 Chlorine trifluoride, Hazard class: 2.3;
Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated
truck. Protect cylinder and labels from physical damage.
The owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations to refill
compressed gas cylinders without the express written permission of the owner. | | Purification Methods | Impurities include chloryl fluoride, chlorine dioxide and hydrogen fluoride. Passed it first through two U-tubes containing NaF to remove HF, then through a series of traps in which the liquid is fractionally distilled. It can be purified via the KF complex; KClF4, formed by adding excess ClF3 to solid KF in a stainless steel cylinder in a dry-box and shaking overnight. After pumping out the volatile materials, pure ClF3 is obtained by heating the bomb to 100-150o and condensing the evolved gas in a -196o trap [Schack et al. Chem Ind (London) 545 1967]. It attacks glass very vigorously. HIGHLY TOXIC. | | Incompatibilities | A powerful oxidizer. Keep away from
acids. Most combustible materials ignite spontaneously
on contact with chlorine trifluoride. Explodes on contact
with organic materials. The liquid can explode if mixed
with halocarbons or hydrocarbons. It reacts violently
with oxidizable materials, finely divided metals and
metal oxides; sand, glass, asbestos, silicon-containing
compounds. Emits highly toxic fumes on contact with
acids. Chlorine trifluoride decomposes above 220C,
forming Thermal decomposition products may include
hydrogen chloride and HF. Reacts violently with water,
forming chlorine gas and hydrofluoric acid. Reacts with
most forms of plastics, rubber, coatings, and resins;
except the highly fluorinated polymers, such as Teflon
and “K el-F.” | | Waste Disposal | Return refillable compressed
gas cylinders to supplier. |
| | CHLORINE TRIFLUORIDE Preparation Products And Raw materials |
|