| | CHLORINE PENTAFLUORIDE Basic information |
| Product Name: | CHLORINE PENTAFLUORIDE | | Synonyms: | CHLORINE PENTAFLUORIDE;chlorinefluoride(clf5);ClF5;Chlorine pentafluoride 99%;Chlorinepentafluoride99%;KNSWNNXPAWSACI-UHFFFAOYSA-N;pentafluoro-λ5-chlorane | | CAS: | 13637-63-3 | | MF: | ClF5 | | MW: | 130.45 | | EINECS: | 237-123-6 | | Product Categories: | | | Mol File: | 13637-63-3.mol | 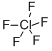 |
| | CHLORINE PENTAFLUORIDE Chemical Properties |
| Melting point | -103°C | | Boiling point | -13,1°C | | density | 1.774 (estimate) | | form | colorless gas | | color | colorless | | Exposure limits | TLV-TWA 2.5 mg(F)/m3 (ACGIH, MSHA,
and OSHA). | | EPA Substance Registry System | Chlorine fluoride (ClF5) (13637-63-3) |
| Hazard Codes | O | | Safety Statements | 17-36/37/39 | | RIDADR | 2548 | | Hazard Note | Oxidising agent | | HazardClass | 2.3 | | HS Code | 2812199000 | | IDLA | 1.7 ppm |
| | CHLORINE PENTAFLUORIDE Usage And Synthesis |
| Chemical Properties | colorless gas; critical temp 142.6°C; enthalpy of vaporization 22.21 kJ/mol; specific conductivity 1.25×10?9 ohm· cm [KIR78] | | Uses | Chlorine pentafluoride does not have anysignificant commercial application. It is usedas a fluorinating and oxidizing agent. | | General Description | A colorless gas with a sweet odor. Toxic by inhalation and an irritant to skin, eyes and mucus membranes. Corrosive. Heavier than air. Under prolonged exposure to fire or intense heat the containers may violently rupture or rocket. Used as an oxidizer in propellants. | | Air & Water Reactions | Reacts with water or moisture in the air to produce corrosive hydrofluoric acid and toxic chloride gas. Interaction with ice at -100°C, or with water vapor above 0°C is extremely vigorous (Christe, K.O. Inorg. Chem. 1972 11, 1220). | | Reactivity Profile | CHLORINE PENTAFLUORIDE is a strong oxidizing agent. Nonflammable, but likely to react vigorously on contact with combustible materials. Reacts violently with lithium, calcium. Emits highly toxic fluoride and chloride fumes when heated to decomposition. Extremely vigorous reaction with water, steam, even ice [Pilipovich D. et al., Inorg. Chem., 1967, 6, p. 1918]. Very vigorous reaction with anhydrous nitric acid even at -100° C [Christie, K. O., Inorg. Chem. 1992, 11, p. 1220]. | | Health Hazard | TOXIC; may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution. | | Health Hazard | Chlorine pentafluoride is a highly toxic gas.It is a severe irritant to the eyes, skin,and mucous membranes. Exposure can causelacrimation, corneal damage, skin burn, andlung damage. Other symptoms are nausea,vomiting, and dyspnea. The liquid is highlycorrosive to skin, causing painful burns.
LC50 value, inhalation (rats): 122 ppm/h. | | Fire Hazard | Substance does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. | | Safety Profile | Poison by inhalation. A
corrosive material. Vigorous reaction in
contact with water or anhydrous nitric acid.
Violent reaction on contact with metals.
When heated to decomposition it emits very
toxic fumes of Cland F-. See also
CHLORINE, FLUORINE, FLUORIDES,
and CHLORINE TRIFLUORIDE. |
| | CHLORINE PENTAFLUORIDE Preparation Products And Raw materials |
|