| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Email: |
marketing@energy-chemical.com |
| Products Intro: |
Product Name:Phenaglycodol
CAS:79-93-6
Purity:NULL Package:10mg;25mg;50mg;5mg Remarks:NULL
|
| Company Name: |
Beijing Biocreative Technology Co., Ltd.
|
| Tel: |
15522676233 |
| Email: |
3007606172@qq.com |
| Products Intro: |
Product Name:Phenaglycodol
CAS:79-93-6
Purity:99% HPLC Package:5.0mg; 10.0mg; 25.0mg; 50.0mg
|
|
| | phenaglycodol Basic information |
| Product Name: | phenaglycodol | | Synonyms: | phenaglycodol;2-(4-chlorophenyl)-3-methyl-butane-2,3-diol;Phenaglycodol: (Ultran: 2-p-chlorophenyl-3-methyl-2,3-butanediol);2-(4-Chlorophenyl)-3-methyl-2,3-butanediol;Acalmid;Acalo;Sinforil;Ultran | | CAS: | 79-93-6 | | MF: | C11H15ClO2 | | MW: | 214.69 | | EINECS: | 201-235-3 | | Product Categories: | | | Mol File: | 79-93-6.mol | 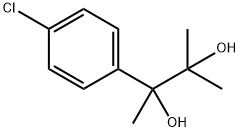 |
| | phenaglycodol Chemical Properties |
| Melting point | 77-78℃ | | Boiling point | 308.17°C (rough estimate) | | density | 1.1153 (rough estimate) | | refractive index | 1.4580 (estimate) | | storage temp. | -20°C Freezer | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Off-White |
| Toxicity | LD50 oral in rat: 832mg/kg |
| | phenaglycodol Usage And Synthesis |
| Originator | Ultran, Lilly ,US ,1975 | | Uses | Phenaglycodol is a propanediol-type tranquilizer that can lead to gynecomastia and urinary steroid excretion in humans that are treated with the drug. | | Definition | ChEBI: Phenaglycodol is an alkylbenzene. | | Manufacturing Process | To a mixture of 460 g of p-chloroacetophenone, 350 ml of ether and 500 ml of water are added 410 g of sodium cyanide, with vigorous stirring. The reaction mixture is cooled to about 5°C to 10°C and 700 ml of concentrated hydrochloric acid are added at such a rate that no hydrogen cyanide is formed and the temperature of the mixture does not rise above 10°C. After the addition of the acid is complete, the reaction mixture is stirred for about three hours at room temperature, and allowed to separate into an aqueous and an organic phase. The organic phase is removed from the aqueous phase, and the aqueous phase and any salt which may have separated in the course of the reaction are washed with about 300 ml of ether. The combined ether washings and organic phase are dried over anhydrous magnesium sulfate, and the ether is removed by evaporation in vacuo at room temperature. The residue is poured with stirring into 800 ml of concentrated hydrochloric acid kept at about 0°C by cooling with solid carbon dioxide. The acid mixture is saturated with gaseous hydrogen chloride at 0°C, and stirred at room temperature overnight. The resulting precipitate of p-chloroatrolactamide is removed by filtration, washed by slurrying with water and dried. After recrystallization from ethanol, p-chloroatrolactamide melts at about 105°C to 107°C.
A mixture of 200 g of p-chloroatrolactamide and 1 liter of 25% sodium hydroxide solution is refluxed with stirring for about sixteen hours. The reaction mixture is then poured over cracked ice and diluted with water to a volume of about 3 liters. The aqueous solution is washed with two 1 liter portions of ether, and acidified with concentrated hydrochloric acid, whereupon a precipitate of p-chloroatrolactic acid forms. The precipitated acid is removed by filtration, and is dissolved in 500 ml of ether, washed with two 250 ml portions of water and dried. The ether is removed by evaporation. pchloroatrolactic acid thus prepared melts at about 117°C to 120°C.
A mixture of 185 g of p-chloroatrolactic acid, 600 ml of ethanol and 60 ml of concentrated sulfuric acid is refluxed for about twelve hours. About half the solvent is then removed by evaporation in vacuo at room temperature, the residue is poured over cracked ice, and diluted with water to a volume of about 2 liters. The ethyl p-chloroatrolactate formed in the reaction is extracted with two 1 liter portions of ether. The combined ether extracts are washed with successive 200 ml portions of water, 5% sodium carbonate solution, and water, and are dried over anhydrous magnesium sulfate. The dried ether solution is subjected to fractional distillation, and the fraction boiling at about 90°C to 100°C at a pressure of 0.1 mm of mercury, is collected. The distillate consists of ethyl p-chloroatrolactate.
To a solution of 2 mols of methylmagnesium iodide in 1.5 liters of ether are added with vigorous stirring 107 g (0.5 mol) of ethyl p-chloroatrolactate. The reaction mixture is stirred for about sixteen hours, and is then decomposed by the addition of about 320 ml of saturated aqueous ammonium chloride solution. After standing, the ether layer is decanted from the mixture and the aqueous phase and the precipitated salts are washed with several 500 ml portions of ether. The combined ether solution and washings are washed with successive 500 ml portions of 5% ammonium chloride solution and water, are dried over anhydrous magnesium sulfate, and are evaporated to dryness in vacuo. The crystalline residue consisting of 2-p-chlorophenyl-3-methyl-2,3butanediol, is recrystallized from a mixture of benzene and petroleum ether.
2-p-chlorophenyl-3-methyl-2,3-butanediol thus prepared melts at about 66°C to 67°C. | | Therapeutic Function | Tranquilizer |
| | phenaglycodol Preparation Products And Raw materials |
|