- Iron(II) Fluoride
-

- $100.00/ kg
-
2023-07-26
- CAS:7789-28-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500t/month
|
| Product Name: | IRON (II) FLUORIDE | | Synonyms: | ironfluoride(fef2);IRON FLUORIDE;FE F2;FERROUS FLUORIDE;Iron(II) fluoride, 99.90%;Ferrousfluoride,anhydrous;Iron (II) fluoride, anhydrous 99.9%;Iron(II)fluoride,anhydrous,99% | | CAS: | 7789-28-8 | | MF: | F2Fe | | MW: | 93.84 | | EINECS: | 232-155-7 | | Product Categories: | metal halide | | Mol File: | 7789-28-8.mol | 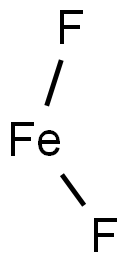 |
| | IRON (II) FLUORIDE Chemical Properties |
| Melting point | >1000°C | | density | 4,09 g/cm3 | | solubility | slightly soluble in H2O; soluble in dilute HF; insoluble in EtOH, ethyl ether | | form | Powder | | color | White | | Specific Gravity | 4.09 | | Water Solubility | Insoluble in water. | | Sensitive | Air Sensitive & Hygroscopic | | Merck | 14,4045 | | Stability: | hygroscopic | | CAS DataBase Reference | 7789-28-8(CAS DataBase Reference) | | EPA Substance Registry System | Iron fluoride (FeF2) (7789-28-8) |
| Hazard Codes | C | | Risk Statements | 34 | | Safety Statements | 26-36/37/39-45 | | RIDADR | UN 3260 8/PG 2 | | WGK Germany | 3 | | F | 3 | | Hazard Note | Corrosive | | TSCA | Yes | | HazardClass | 6.1 | | PackingGroup | III | | HS Code | 2826199090 |
| | IRON (II) FLUORIDE Usage And Synthesis |
| Preparation | Anhydrous iron(II) fluoride may be prepared by passing hydrogen fluoride gas over iron at a high temperature:
2HF + Fe → FeF2 + H2
Alternative methods of preparation of anhydrous salt involve the reduction of iron(III) fluoride with hydrogen; or by passing fluorine gas over anhydrous iron(II) chloride in the cold:
2FeF3 + H2 → 2FeF2 + 2HF
FeCl2 + F2 → FeF2 + Cl2
The tetrahydrate may be prepared by dissolving iron metal in aqueous hydrofluoric acid.
| | Chemical Properties | brown fine crystalline powder | | Chemical Properties | Iron(II) fluoride is prepared by passing hydrogen fluoride over iron at red heat
or over iron(II) chloride at a somewhat lower temperature. It is sparingly soluble in water with some hydrolysis, and is insoluble in alcohol, ether and benzene. The boiling point is
about 1100°C and the melting point only a little below this temperature. | | Physical properties | White tetragonal crystal; density 4.09g/cm3; melts at 1100°C; slightly soluble in water; insoluble in ethanol and ether; dissolves in dilute hydrofluoric acid. Tetrahydrate crystals are hexagonal shape; density 2.20g/cm3; decomposes at 100°C.
. | | Uses | Iron(II) fluoride is used for proteomics research applications. It is also a chemical intermediate. | | Uses | Iron(II) fluoride is used as a catalyst in organic fluorination reactions. Other applications are in ceramics; and in the preparation of fluoride salts of other metals. | | Structure and conformation | Iron(II) fluoride crystallizes in
the rutile structure, the iron atom being surrounded tetragonally91 by 4F- at 2·12 ? and 2F-
at 1·99 ?. Iron(II) fluoride is reduced to iron by hydrogen at red heat, but is stable to
hydrogen at 400°—and can be prepared by reduction of iron(III) fluoride with hydrogen at
this temperature. It reacts violently when heated with metals such as sodium and aluminium,
but undergoes no visible reaction with bromine, iodine or sulphur. The ammoniates
FeF2.5NH3,H20, FeF2.NH3.H20 and FeF2.0·5NH3.H2O are obtained in the reactions
of gaseous ammonia with the tetrahydrate at various temperatures. |
| | IRON (II) FLUORIDE Preparation Products And Raw materials |
|