|
|
| | 7-Aminocephalosporanic acid Basic information |
| | 7-Aminocephalosporanic acid Chemical Properties |
| Melting point | >300 °C (lit.) | | alpha | 94 º (c=0.5, KH2PO4, PH 7) | | Boiling point | 560.6±50.0 °C(Predicted) | | density | 1.4667 (rough estimate) | | vapor pressure | 0Pa at 20℃ | | refractive index | 1.5650 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO (Very Slightly, Heated) | | pka | 2.59±0.50(Predicted) | | form | Crystalline Powder | | color | Off-white to beige | | optical activity | [α]19/D +90°, c = 0.5 in KH2PO4/trace NaOH | | Water Solubility | 409.6mg/L(22.99 ºC) | | Merck | 14,434 | | BRN | 622638 | | Stability: | Hygroscopic | | InChIKey | HSHGZXNAXBPPDL-HZGVNTEJSA-N | | LogP | -3.4 at 20℃ | | CAS DataBase Reference | 957-68-6(CAS DataBase Reference) |
| | 7-Aminocephalosporanic acid Usage And Synthesis |
| Description | The abbreviation for 7-aminocephalosporanic acid is 7-ACA. It is a white or almost white crystalline powder and serves as a crucial nucleus in the production of cephalosporin antibiotics. By utilizing chemical transformations at positions 7 and 3 of the nucleus, numerous cephalosporins can be synthesized, including cefazolin sodium, cefotaxime sodium, ceftriaxone sodium, cefoperazone sodium, sodium ceftazidime, and cefuroxime sodium.
Cephalosporin antibiotics (Cephalosporins) are a cluster of broad-spectrum semisynthetic antibiotics, they all contain the nucleus of 7-aminocephalosporanic acid (7-ACA), with different groups in the 3 and 7 carbon atoms, forming various cephalosporins with different antibacterial activity and pharmacokinetic characteristics. Cephalosporin antibiotics have broad antibacterial spectrum,strong antibacterial effect, and fewer allergic reactions, and only part of the cross allergic with penicillin and they have varying degrees of stability on the β-lactamase . Cephalosporins have fast development, and many families, they are divided into four generation cephalosporins according to the anti-microbial dynamic "generations" .
They are the same as penicillin, cephalosporins contain β lactam ring , which is necessary to achieve antibacterial efficacy. But penicillins are 6-amino penicillin acid (6-aminopenicillanic acid, 6-APA) derivatives, and cephalosporins nucleus is 7-aminocephalosporanic acid (7-aminocephalosporamic acid, 7-ACA), the latter is from cephalosporin C, it is a fermentation product of Cephalosporium acremonium.
7-aminocephalosporanic acid has a dihydro-thiazine ring (A) and aβ-lactam ring (B), it has a resistant effect on the class of staphylococcus aureus penicillinase. Modifying this nucleus with different side chains can form the entire series of cephalosporin antibiotics. Modifying β-lactam bit 7 (R1), can make antimicrobial efficacy and stability within the β-lactamase change. At 3 bit on-dihydro-thiazine ring substitution (R2), the influence of effect on drug metabolism and pharmacokinetic properties is more staggering than that of antibacterial effect.
Cephamycins are associated with cephalosporin C in chemical structure, the main difference is that they have a 7-α-methoxy group (R3), so that the stability to certain β-lactamase is improved . Cephalosporin is a derivative cephalosporin C which is produced by Streptomyces . Cefotetan is an semi-synthetic derivative of organic mycin G, it is a product of Streptomyces organonensis. Cefmetazole is a semi-synthetic product of 7-aminocephalosporanic acid.
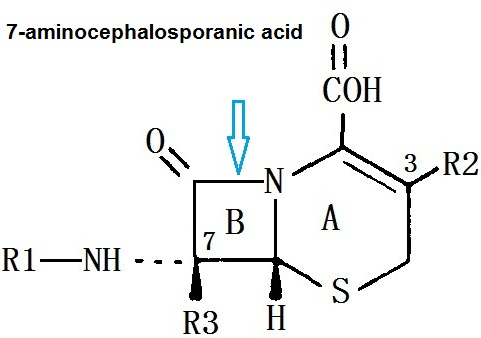
Figure 1 the chemical structure of the 7-aminocephalosporanic acid | | Chemical Properties | Off-white to beige crystalline powder | | Uses | 7-Aminocephalosporanic Acid (Cefoperazone EP Impurity E) is the starting material for semi-synthetic cephalosporins. Obtained by mild acid hydrolysis of cephalosporin C. | | Uses | Potent antibacterial that inhibits bacterial transpeptidase and β-lactamase activity in Staphylococcus aureus. | | Definition | ChEBI: 7-Aminocephalosporanic acid is the alpha,beta-unsaturated monocarboxylic acid that is the active nucleus for the synthesis of cephalosporins and intermediates. It is functionally related to a cephalosporanic acid. It is a tautomer of a 7beta-aminocephalosporanic acid zwitterion. | | Preparation | The synthesis of 7-Aminocephalosporanic acid involves esterification of semi-synthetic cephalosporin-cephalosporin C with trimethylchlorosilane, followed by phosphorus pentachloride chloride and butanol etherifying. The final product is obtained through hydrolysis. The yield from cephalosporin C sodium to 7-ACA is approximately 50%.
DOI: 10.3390/jof8050450 | | General Description | The chemical class of 7-aminocephalosporanic acid is Beta-Lactams. This class includes several antibiotic families, such as penicillins, cephalosporins, carbapenems, and monobactams. The Beta-lactam ring is part of the core structure of these families, which is why they are also referred to as Beta-lactam antibiotics. | | Flammability and Explosibility | Not classified |
| | 7-Aminocephalosporanic acid Preparation Products And Raw materials |
|