- Cemastin
-

- $1.22 / 1KG
-
2022-02-25
- CAS:153-61-7
- Min. Order: 300g
- Purity: 99%
- Supply Ability: 3tons
|
| | Cephalothin Basic information |
| Product Name: | Cephalothin | | Synonyms: | CEPHALOTHIN;(6R,7R)-3-(Acetoxymethyl)-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6R,7R)-3-(Acetoxymethyl)-8-oxo-7-[2-(2-thienyl)acetylamino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;C07761;(6R,7R)-3-[(acetyloxy)Methyl]-8-oxo-7-[2-(thiophen-2-yl)acetaMido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6R,7R)-3-[(Acetyloxy)Methyl]-8-oxo-7-[[2-(2-thienyl)acetyl]aMino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid;(6R)-3-acetoxyMethyl-8-oxo-7t-(2-thiophen-2-yl-acetylaMino)-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid, 3-[(acetyloxy)methyl]-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-, (6R,7R)- | | CAS: | 153-61-7 | | MF: | C16H16N2O6S2 | | MW: | 396.44 | | EINECS: | 205-815-7 | | Product Categories: | KEFLIN;Amines;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 153-61-7.mol | 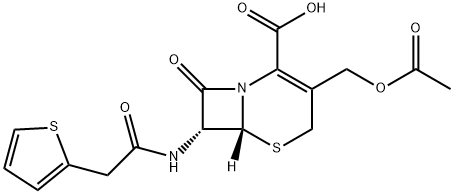 |
| | Cephalothin Chemical Properties |
| Melting point | 160-161 ºC | | alpha | D20 +50° (c = 1.03 in acetonitrile) | | Boiling point | 757.2±60.0 °C(Predicted) | | density | 1.4162 (rough estimate) | | refractive index | 1.6510 (estimate) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | DMSO (Sparingly), Dioxane (Slightly), Methanol (Slightly) | | form | Solid | | pka | pKa 2.5 (Uncertain) | | color | White to Off-White | | Water Solubility | 158 MG/L | | λmax | 263nm(H2O)(lit.) | | Merck | 14,1982 | | CAS DataBase Reference | 153-61-7(CAS DataBase Reference) | | EPA Substance Registry System | Cephalothin (153-61-7) |
| | Cephalothin Usage And Synthesis |
| Description | Cephalothin is a semisynthetic, beta-lactam, first-generation cephalosporin antibiotic with bactericidal activity. Cephalothin binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. PBPs participate in the terminal stages of assembling the bacterial cell wall, and in reshaping the cell wall during cell division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. | | Chemical Properties | White Solid | | Originator | Keflin,Lilly,US,1964 | | Uses | A first generation cephalosporin antibiotic with a wide range antibacterial activity. It is effective against gram-positive and gram-negative bacteria, and has been tested in respiratory disease and neuromuscular junction studies.
Cephalothin is most effective against Gram-positive cocci and is also effective against several Gram-negative organisms. First generation cephalosporins are used primarily to treat skin and soft tissue infections caused by Staphylococcus and Streptococcus species. Cephalothin is ineffective against enterococci and evidence of in vitro sensitivity should be discounted. | | Definition | ChEBI: Cefalotin is a semisynthetic, first-generation cephalosporin antibiotic with acetoxymethyl and (2-thienylacetyl)nitrilo moieties at positions 3 and 7, respectively, of the core structure. Administered parenterally during surgery and to treat a wide spectrum of blood infections. It has a role as an antimicrobial agent and an antibacterial drug. It is a semisynthetic derivative, a beta-lactam antibiotic allergen, a cephalosporin, a carboxylic acid, a member of thiophenes and an azabicycloalkene. It is a conjugate acid of a cefalotin(1-). | | Manufacturing Process | 7-(2'-Thienylacetamido)cephalosporanic acid sodium salt may be produced
from 2-thienylacetyl chloride, obtainable by treatment of 2-thienylacetic acid
[Ernst, Berichte, 19 (1886) 3281] with thionyl chloride in a conventional
manner. The 2-thienylacetyl chloride is then reacted with 7-
aminocephalosporanic acid and then converted to the sodium salt using
sodium hydroxide. | | Brand name | Keflin
(Lilly); Seffin (GlaxoSmithKline). | | Therapeutic Function | Antibacterial | | Acquired resistance | Cephalothin is relatively susceptible to β-lactamases. Enterobacter, Klebsiella and Citrobacter species have acquired resistance by chromosomal constitutively produced βlactamases that are not inhibited by clavulanic acid. Bacteroides species develop resistance to β-lactams both by plasmids and chromasomally; however Bacteroides resistance remains susceptible to clavulanic acid. P aeruginosa is resistant to first and second generation cephalosporins because of problems with cell permeability/uptake, porin channels and drug efflux. Stenotrophomonas maltophila and Aeromonas species can be resistant through effects on porin channels and drug efflux, while S aureus can also be resistant due to drug efflux. | | Mechanism of action | Inhibits bacterial cell wall synthesis by binding to one or more of the penicillin-binding proteins (PBPs) which in turn inhibits the final transpeptidation step of peptidoglycan synthesis in bacterial cell walls, thus inhibiting cell wall biosynthesis. Bacteria eventually lyse due to ongoing activity of cell wall autolytic enzymes (autolysins and murein hydrolases) while cell wall assembly is arrested. | | Clinical Use | Cephalothin is a relatively short acting first generation cephalosporin that is administered injectably. Although cephalothin is not approved for use in any animal species in the United States, the drug is used clinically in veterinary medicine. Cephalothin can cause nephrotoxicity. | | Side effects | Manifestations may include urticarial or maculopapular rash, bronchospasm, and drug fever. Anaphylaxis, including severe hypotension and cardiac arrest, is reported. Rare cases of renal insufficiency associated with cephalothin may be hypersensitivity-mediated since fever, eosinophilia, and rash are often also present.
https://www.drugs.com |
| | Cephalothin Preparation Products And Raw materials |
|