- Fluvoxamine
-

- $0.00 / 25KG
-
2024-03-29
- CAS:54739-18-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Fluvoxamine
-
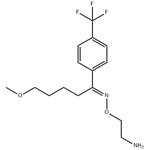
- $0.00 / 10mg
-
2023-11-01
- CAS:54739-18-3
- Min. Order: 1mg
- Purity: 99% HPLC
- Supply Ability: 100kg
- Fluvoxamine USP/EP/BP
-
- $1.10 / 1g
-
2021-07-27
- CAS:54739-18-3
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
|
| | Fluvoxamine Basic information |
| Product Name: | Fluvoxamine | | Synonyms: | 2-[[5-methoxy-1-[4-(trifluoromethyl)phenyl]-pentylidene]amino]oxyethanamine;(1E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentanal O-(2-aminoethyl)oxime;(E)-ω-Methoxy-4'-(trifluoromethyl)valerophenone O-(2-aminoethyl)oxime;δ-Methoxy-4'-(trifluoromethyl)valerophenone (E)-O-(2-aminoethyl)oxime;Fluvoxamine�See: F603500;Fluvoxamine
Also See: F603500
;FLUVOXAMINE;(E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]-1-pentanoneo-(2-aminoethyl)oxime | | CAS: | 54739-18-3 | | MF: | C15H21F3N2O2 | | MW: | 318.33 | | EINECS: | 611-193-1 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 54739-18-3.mol | 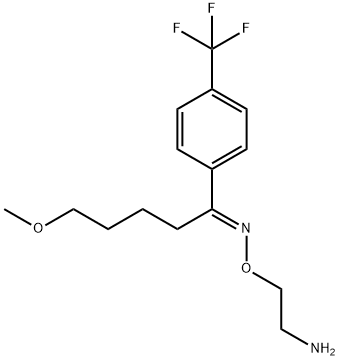 |
| | Fluvoxamine Chemical Properties |
| Melting point | 120-122.5°C | | Boiling point | 370.6±52.0 °C(Predicted) | | density | 1.16±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) | | form | Oil | | pka | pKa 8.7 (Uncertain) | | color | Colourless | | CAS DataBase Reference | 54739-18-3(CAS DataBase Reference) | | NIST Chemistry Reference | Fluvoxamine(54739-18-3) |
| | Fluvoxamine Usage And Synthesis |
| Chemical Properties | Colourless Oil | | Originator | Floxyfral, Kali-Duphar ,Switz. ,1983 | | Uses | A selective serotonin reuptake inhibitor (SSRI) used as an anti-depressant. | | Definition | ChEBI: Fluvoxamine is an oxime O-ether that is benzene substituted by a (1E)-N-(2-aminoethoxy)-5-methoxypentanimidoyl group at position 1 and a trifluoromethyl group at position 4. It is a selective serotonin reuptake inhibitor that is used for the treatment of obsessive-compulsive disorder. It has a role as an antidepressant, a serotonin uptake inhibitor and an anxiolytic drug. It is a 5-methoxyvalerophenone O-(2-aminoethyl)oxime and a member of (trifluoromethyl)benzenes. It is functionally related to a (trifluoromethyl)benzene. | | Manufacturing Process | 20.4 mmol (5.3 g) of 5-methoxy-4'-trifluoromethylvalerophenone (MP 43°C to 44°C), 20.5 mmol (3.1 g) of 2-aminooxyethylamine dihydrochloride and 10 ml of pyridine were refluxed for 15 hr in 20 ml of absolute ethanol. After evaporating the pyridine and the ethanol in vacuo, the residue was dissolved in water. This solution was washed with petroleum ether and 10 ml of 50% sodium hydroxide solution were then added. Then three extractions with 40 ml of ether were carried out. The ether extract was washed successively with 20 ml of 5% sodium bicarbonate solution and 20 ml of water. After drying on sodium sulfate, the ether layer was evaporated in vacuo. Toluene was then evaporated another three times (to remove the pyridine) and the oil thus obtained was dissolved in 15 ml of absolute ethanol. An equimolar quantity of maleic acid was added to the solution and the solution was then heated until a clear solution was obtained. The ethanol was then removed in vacuo and the residue was crystallized from 10 ml of acetonitrile at +5°C. After sucking off and washing with cold acetonitrile, it was dried in air. The MP of the resulting compound was 120°C to 121.5°C. | | Brand name | Luvox (Solvay Pharmaceuticals);Faverin. | | Therapeutic Function | Antidepressant | | General Description | The E-isomer of fluvoxamine (Luvox) can fold after protonation to the β-arylamine–like grouping. Here,the “extra” hydrophobic group is aliphatic. | | Hazard | A poison. | | Biological Activity | fluvoxamineis is an antidepressant which pharmacologically functions as a selective serotonin reuptake inhibitor. serotonin, also known as 5-ht, is amonoamine neurotransmitter with various physiological functions such as mood, appetite, and sleep[1]. | | Mechanism of action | Fluvoxamine is a highly selective inhibitor of 5-HT reuptake at the presynaptic membrane. Potency data from
in vitro affinity studies suggest that fluvoxamine is less potent than the other SSRIs (e.g., paroxetine,
sertraline, and citalopram). Its mechanism of action is similar to that of the other SSRIs. Fluvoxamine appears
to have little or no effect on the reuptake of NE or dopamine. In vitro studies have demonstrated that
fluvoxamine possesses virtually no affinity for other neuroreceptors. Its onset of action is similar to the other
SSRIs (2–4 weeks). | | Pharmacokinetics | Fluvoxamine is well absorbed, with a bioavailability of approximately 50%, probably because of first-pass
metabolism. At steady-state doses, fluvoxamine demonstrates nonlinear pharmacokinetics over a
dosage range of 100 to 300 mg/day, which results in higher plasma concentrations at higher doses than
would be predicted by lower-dose kinetics (single dose, 15 hours; multiple dosing, 22 hours). Food does not
significantly affect oral bioavailability. The mean apparent volume of distribution for fluvoxamine reflects its
lipophilic nature, extensive tissue distribution, and protein binding. Fluvoxamine is distributed into breast
milk. Fluvoxamine is preferentially metabolized by CYP2D6 in the liver by O-demethylation to its alcohol
metabolite, which subsequently is oxidized to a carboxylic acid. Oxidative deamination and nine other
metabolites have been identified, none of which shows significant pharmacological activity. | | Clinical Use | Fluvoxamine is approved for use in obsessive-compulsive disorders. | | Side effects | The adverse effects for fluvoxamine include symptoms of drowsiness, nausea or vomiting, abdominal pain,
tremors, sinus bradycardia, and mild anticholinergic symptoms. Toxic doses could produce seizures and
severe bradycardia. | | in vitro | fluvoxamine effectively inhibited 5-ht uptake by blood platelets and brain synaptosomes [1].fluvoxamine (10 mg/kg) increased [5-ht]ex levels in all brain areas and increased [da]ex levels in the striatum. fluvoxamine (10 mg/kg) in combination with of quetiapine (10 mg/kg) increased [da]ex and [5-ht]ex levels in all brain areas when compared with baseline. the combination produced a significant increase of [da]ex levels in the prefrontal cortex and thalamus whereas neither quetiapine nor fluvoxamine in monotherapy affected [da]ex levels [2]. fluvoxamine induced antiallodynic effects on neuropathic pain via descending 5-ht fibers and spinal 5-ht2a or 5-ht2c receptors, and the antinociception on acute mechanical pain via 5-ht3 receptors [3]. | | in vivo | single administration of fluvoxamine (10 and 30 mg/kg, i.p.) dose-dependently enhanced synaptic efficacy in the hippocampo-mpfc pathway [4]. fluvoxamine (10 and 30 mg/kg, i.p.) treatment suppressed long-term potentiation (ltp) in the hippocampal ca1 field of anesthetized rats. nan-190 (0.5 mg/kg, i.p), the 5-ht(1a) receptor antagonist, completely reversed the fluvoxamine (30 mg/kg, i.p.) suppression of ltp [5]. | | Drug interactions | In vitro studies have shown fluvoxamine to be a potent inhibitor of CYP1A2, to inhibit CYP3A4 and CYP2C19,
and to weakly inhibit CYP2D6. The bioavailability of fluvoxamine is significantly decreased in smokers
compared with nonsmokers, possibly because of induction of CYP1A metabolism of fluvoxamine. Therefore,
interactions with drugs that inhibit CYP1A2 also should be considered (e.g., theophylline and caffeine). | | references | [1]. claassen v,davies je,hertting g,placheta p. fluvoxamine, a specific 5-hydroxytryptamine uptake inhibitor.br j pharmacol.1977 aug;60(4):505-16.
[2]. denys d1,klompmakers aa,westenberg hg. synergistic dopamine increase in the rat prefrontal cortex with the combination of quetiapine and fluvoxamine.psychopharmacology (berl).2004 nov;176(2):195-203. epub 2004 may 11.
[3]. honda m1,uchida k,tanabe m,ono h. fluvoxamine, a selectiveserotoninreuptake inhibitor, exerts its antiallodynic effects on neuropathic pain in mice via 5-ht2a/2c receptors.neuropharmacology.2006 sep;51(4):866-72. epub 2006 jul 17.
[4]. ohashi s1,matsumoto m,otani h,mori k,togashi h,ueno k,kaku a,yoshioka m. changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine.brain res.2002 sep 13;949(1-2):131-8.
[5]. kojima t1,matsumoto m,togashi h,tachibana k,kemmotsu o,yoshioka m. fluvoxamine suppresses the long-term potentiation in the hippocampal ca1 field of anesthetized rats: an effect mediated via 5-ht1a receptors.brain res.2003 jan 3;959(1):165-8. |
| | Fluvoxamine Preparation Products And Raw materials |
|