- 2-Butanone oxime
-

- $0.00 / 200Kg/Drum
-
2024-09-23
- CAS:96-29-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year
- 2-Butanone oxime
-

- $0.00 / 10ml
-
2024-09-18
- CAS:96-29-7
- Min. Order: 10ml
- Purity: 99%
- Supply Ability: 50L
|
| Product Name: | 2-Butanone oxime | | Synonyms: | (2E)-2-Butanone oxime;2-Butoxime;Aron M 1;Butan-2-one, oxime;butan-2-oneoxime;Butanoxime;ethylmethylcetoxime;Ethyl-methylketonoxim | | CAS: | 96-29-7 | | MF: | C4H9NO | | MW: | 87.12 | | EINECS: | 406-930-7 | | Product Categories: | Organics | | Mol File: | 96-29-7.mol | 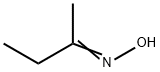 |
| | 2-Butanone oxime Chemical Properties |
| Melting point | -30 °C | | Boiling point | 59-60 °C15 mm Hg(lit.) | | density | 0.924 g/mL at 25 °C(lit.) | | vapor density | 3 (vs air) | | vapor pressure | <8 mm Hg ( 20 °C) | | refractive index | n20/D 1.442(lit.) | | Fp | 140 °F | | solubility | water: soluble100g/L at 25°C | | form | Liquid | | pka | pK1:12.45 (25°C) | | color | Clear colorless to pale yellow | | Water Solubility | 114 g/L (20 ºC) | | BRN | 1698241 | | Dielectric constant | 3.4(20℃) | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. May react with strong acids to form an explosive material. | | InChIKey | WHIVNJATOVLWBW-SNAWJCMRSA-N | | LogP | 0.63 at 25℃ | | CAS DataBase Reference | 96-29-7(CAS DataBase Reference) | | NIST Chemistry Reference | 2-Butanone, oxime(96-29-7) | | EPA Substance Registry System | Methyl ethyl ketoxime (96-29-7) |
| | 2-Butanone oxime Usage And Synthesis |
| Oxime oxygen scavenger | Oxime compounds (dimethyl ketone oxime, methyl ethyl ketoxime (butanone oxime), acetaldehyde oxime) knows as a novel oxygen scavenger is disclosed in the U.S. and patented by Drew Chemical Company in 1984. It displays low toxicity, efficiency, fast-performance, and a blunt protective effects. In Europe and other developed countries it has been widely applied, and China it is also successfully developed in the nineties, and has been successful in promotion.
1. Oxygen scavenging performance: oxime compound is an organic compound with an oxime group. Oxime compounds are currently used for boiler shutdown protection and oxygen mainly acetaldehyde oxime, dimethyl ketone oxime (acetone oxime) and methyl ethyl ketone oxime. Oxime compounds have a strong reduction, easy to react with oxygen. When put in a wide temperature and pressure ranges, oximes has good oxygen scavenging performance. The optimum temperature range is 138~336 ℃, and pressure range is 0.3~13.7Mpa. According to comparative experiments, under the same conditions, the rate of oxygen and oxygen efficiency oximes is higher than that of hydrazine.
2. corrosion and passivation: oximes can restore high iron and copper oxide into suboxide, which can be a good solution in the steel magnetic oxide film formed on the surface of the metal surface passivation plays well, corrosion inhibition. Wherein dimethylketoximino is the best, using the minimum amount required. According to comparative experiments, oxime compounds having the same passivation, corrosion inhibition hydrazine, can significantly reduce the iron content in solution at high temperature and pressure conditions. The steel has a protective effect, among which the dimethylketoximino is best, which requires the least amount . Meanwhile, oxime compounds have cleaning actions to copper corrosion products deposited in the pipeline, economizer, etc., which is in the initial period of oximes. This is the reason why furnace copper water content is significantly higher.
3. Volatile: the volatile degree of oxime compounds is higher than that of hydrazine, DEHA, morpholine, cyclohexylamine, etc. It is close to the volatility of NH3. When the steam condenses, highly volatile oxygen scavenger will has a certain amount of condensation agent which is dissolved in water, therefore, helpful to protect the condensate system metal material.
4. decomposition: By experiments under the high temperature and pressure conditions, the decomposition products of oxime compound is NH3, N2, H2O, trace of acetic acid, formic acid produces, no adverse effects on water vapor system.
5. low toxicity: based on the data comparison of LD50, the LD50 for hydrazine is 290mg/kg, acetaldehyde oxime is 1900mg/kg, methyl ethyl ketone oxime is 2800mg/kg, dimethylket oximino 5500mg/kg. So the toxicity of hydrazine is very strong, and toxicity of oxime compound is very small. It belongs to low toxicity compounds. Test through the skin and mucous membrane contact with oxygen scavengers showed no significant oximes oxygen scavenger irritation and damage, but hydrazine causes damage of skin irritation, erosion, mucosal hyperemia.
The above information is edited by the Chemicalbook of Tian Ye.
| | Chemical Properties | Colorless oily liquid. Melting point-29.5 ℃. Boiling point 152-153 ℃, 59-60 ℃ (2kPa), the relative density is 0.9232 (20/4 ℃), and refractive index 1.4410. With alcohol, ether immiscibility, dissolved in 10 parts of water.
| | Uses | Methyl ethyl ketoxime is mainly used as anti-skinning agent and silicon curing agent for alkyd resin coatings. It is used as an antioxidant to prevent skin formation, which is better than butyraldehyde oxime and cyclohexanone oxime.
used in organic synthesis.
For a variety of oil-based paint, alkyd paint, epoxy paint, such as esters during storage and transportation of anti-skinning process, also used as a curing agent silicon. | | Preparation | With suitable precautions, to 1 liter of the sodium hydroxylamine disulfonate solution from Preparation 2-1 (approx. 1.2 moles) is added 72 gm (1 mole) of methyl ethyl ketone. The mixture is warmed to 70°C. Then the reaction flask is wrapped with insulation and allowed to cool slowly for 12 hr.
After neutralization with 48% sodium hydroxide solution, the oxime is extracted from the reaction mixture with benzene. The benzene solution is distilled fractionally. The product distills between 152° and 154°C; yield, 65 gm (75%).
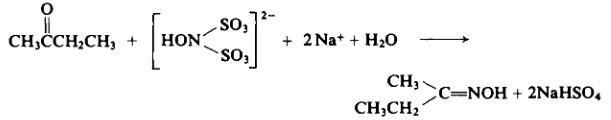
| | Chemical Properties | 2-Butanone oxime is a colorless or light yellow oily transparent liquid that has strong complexation with metal ions and is volatile in the air. It can react with hydrochloric acid and sulfuric acid to form methyl ethyl ketone. | | Uses | 2-Butanone oxime was employed as reagent and solvent in the syntheses of ketoimine and 2,4-dipyridyl-1,3,5-triazapentadiene palladium(II) complexes. It was also used in the synthesis of novel acetaldiimine cobalt complex, [CoI2{((CH3CH2)(CH3)C=NO)2C(CH3)2}]. Methyl ethyl ketoxime is used primarily as an antiskinning agent in alkyd coating resins | | Preparation | 2-Butanone oxime is obtained by the reaction of butanone and hydroxylamine hydrochloride. It can also be synthesized by the reaction between butanone and hydroxylamine sulfate. | | General Description | Clear colorless liquid with a musty odor. | | Air & Water Reactions | Highly flammable. Water soluble. | | Reactivity Profile | 2-Butanone oxime is sensitive to heat. Has exploded at least twice when heated in the presence of acidic impurities [Chem. Eng. News, 1974, 52(35), 3]. Reacts with oxidizing agents. Mixtures with strong acids may explode. Reacts with sulfuric acid to form an explosive product . | | Fire Hazard | 2-Butanone oxime is combustible. | | Flammability and Explosibility | Not classified | | Safety Profile | Poison by
intraperitoneal route. Moderately toxic by
subcutaneous route. May explode if heated.
Reacts with sulfuric acid to form an
explosive product. When heated to
decomposition it emits toxic fumes of NOX. | | Properties and Applications |
|
TEST ITEMS
|
SPECIFICATION
|
|
APPEARANCE
|
COLORLESS TRANSPARENT CLEAR OILY LIQUID,FLAMMABLE
|
|
GRADE
|
EXCELLENT GRADE
|
|
MEKO CONTENT
|
99.7%min
|
|
WATER CONTENT
|
0.04%
|
|
ACID NUMBER (KOH mg/g)
|
0.05 max
|
|
CHROMA NO. (PLATINUM-COBALT)
|
2 max
|
|
| | 2-Butanone oxime Preparation Products And Raw materials |
|