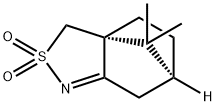
(+)-10-CAMPHORSULFONIMINE synthesis
- Product Name:(+)-10-CAMPHORSULFONIMINE
- CAS Number:107869-45-4
- Molecular formula:C10H15NO2S
- Molecular Weight:213.3
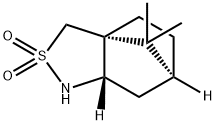
108448-77-7
254 suppliers
$6.00/1g

107869-45-4
80 suppliers
$13.00/1g
Yield:107869-45-4 32 mg
Reaction Conditions:
with NADP in aq. phosphate buffer at 20; pH=8;Enzymatic reaction;
Steps:
4 Isolation and Identification of (+)-camphorsultam Oxidation Products. To isolate compounds 17
To isolate compounds 17, a large scale reaction (250 mE) was set up with P450 mutant III-H2 (2 tM) in 50 mM phosphate buffer (pH 8.0) in the presence of (+)-10,2- camphorsultam (54 mg, final conc.: 1 mM), PTDH at 2 tM, NADP at 150 tM, and sodium phosphite at 50 mM. The mixture was stirred overnight at room temperature. After removal of the enzyme through filtration, the filtrate was loaded on a Cl 8 resin column and the hydroxylated products eluted with acetonitrile. The eluate was dried with Na2504, concentrated in vacuum, and purified by flash chromatography (gradient from 10 to 40% ethyl acetate in hexanes) to afford 17 (32 mg). Compound 17 (camphorsulfonimine). ‘H NMR (500 MHz, CDC13): ?=0.92 (s, 3 H), 1.13 (s, 3 H), 1.50 (m,1H),1.83(m,1H),2.07-2.15(m,2H),2.30(m,1H),2.43 (d, 1 H, J=18.8 Hz), 2.82 (m, 1 H), 3.02 (d, 1 H, J=13.2 Hz), 3.23 (d, 1 H, J=13.2 Hz); ‘3C NMR (100 MHz, CDC13):?=18.9, 19.4, 26.6, 28.3, 35.9, 44.5, 47.9, 49.4, 64.5, 195.1; MS (ESI) calcd for C,QH,6N025 [M+H]/z: 214.09. found:214.42.
References:
US9273342,2016,B2 Location in patent:Page/Page column 45
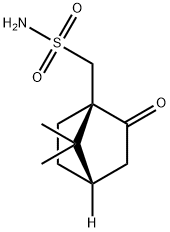
72597-34-3
50 suppliers
$36.79/1g

107869-45-4
80 suppliers
$13.00/1g
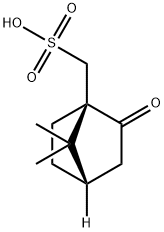
35963-20-3
474 suppliers
$6.00/25g

107869-45-4
80 suppliers
$13.00/1g
39262-22-1
150 suppliers
$8.00/1g

107869-45-4
80 suppliers
$13.00/1g