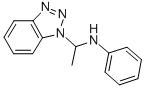
ALPHA-METHYL-N-PHENYL-1H-BENZOTRIAZOLE-1-METHANAMINE synthesis
- Product Name:ALPHA-METHYL-N-PHENYL-1H-BENZOTRIAZOLE-1-METHANAMINE
- CAS Number:122062-68-4
- Molecular formula:C14H14N4
- Molecular Weight:238.29
95-14-7
847 suppliers
$6.00/100g

75-07-0
409 suppliers
$14.00/5mL

62-53-3
629 suppliers
$10.00/1g

122062-68-4
11 suppliers
inquiry
Yield:-
Reaction Conditions:
in diethyl ether at -20 - 0;
Steps:
4
In the published manner (J. Org. Chem. 1995, 60, 3993), benzotriazole (11.9 g, 100 mmol) and aniline (9.1 ml, 100 mmol) were dissolved in diethyl ether (200 ml), and acetaldehyde (6.1 ml, 110 mmol) was added dropwise thereto on ice. The reaction mixture was stirred at room temperature for 15 minutes and then allowed to stand overnight at -20°C. The crystallized product was collected by filtration, washed with diethyl ether and then dried to give α-methyl-N-phenyl-1H-benzotriazole-1-methanamine (22.3 g) as a white crystal. The resulting α-methyl-N-phenyl-1H-benzotriazole-1-methanamine (2.31 g, 10 mmol) and 9-vinylcarbazole (1.93 g, 10 mmol) were suspended in chloroform (10 ml), to which p-toluenesulfonic acid monohydrate (20 mg, 0.105 mmol) was then added and stirred at room temperature for 2 hours. After addition of water, the reaction mixture was extracted with chloroform. The organic layer was washed with 10% aqueous sodium carbonate and water, and then concentrated. The residue was applied to silica gel column chromatography (developing solvent: ethyl acetate/hexane = 1/10) to give the titled compound (644 mg, yield over 2 steps: 20%) as a white crystal.1H NMR (CDCl3) δ 1.27 (d, J = 6.3 Hz, 3H), 2.04-2.16 (m, 1H), 2.45 (q, J= 11.8 Hz, 1H), 3.74-3.85 (m, 1H), 3.90 (br s, 1H), 6.13 (dd, J = 11.9, 6.4 Hz, 1H), 6.47 (t, J = 8.2 Hz, 1H), 6.63 (d, J= 8.3 Hz, 2H), 6.86-6.88 (m, 1H), 7.04 (t, J= 7.7 Hz, 1H), 7.15 (dt, J= 7.3, 1.4 Hz, 2H), 7.22-7.30 (m, 1H), 7.40-7.60 (m, 2H), 8.08-8.15 (m, 2H)Benzotriazole (5.0 g, 4.20 mmol) and aniline (3.91 g, 4.20 mmol) were dissolved in diethyl ether (85 ml), and acetaldehyde (2.03 g, 4.62 mmol) was added dropwise thereto on ice. The reaction mixture was stirred at room temperature for 15 minutes and then allowed to stand overnight at -20°C. The crystallized product was collected by filtration, washed with diethyl ether and then dried to give α-methyl-N-phenyl-1H-benzotriazole-1-methanamine (8.7 g) as a white crystal. The resulting α-methyl-N-phenyl-1H-benzotriazole-1-methanamine (2.86 g, 12 mmol) and vinylphthalimide (2860 mg, 12.0 mmol) were suspended in chloroform (10 ml), to which p-toluenesulfonic acid monohydrate (190 mg, 1.0 mmol) was then added and stirred at room temperature for 10 hours. After addition of water, the reaction mixture was extracted with chloroform. The organic layer was washed with saturated aqueous sodium carbonate and water, and then concentrated. The residue was applied to silica gel column chromatography to give the titled compound (1.03 g, yield over 2 steps: 25%) as a white crystal.1H-NMR (CDCl3) δ 1.21 (d, J = 6.2 Hz, 3H), 2.14 (m, 1H), 2.41 (d, J = 13.7 Hz, 1H), 3.41 (m, 1H), 4.15 (s, 1H), 6.22 (dd, J = 4.4, 3.2 Hz, 1H), 6.64-6.72 (m, 2H), 6.88 (d, J = 7.1 Hz, 1H), 7.00 (t, J = 7.1 Hz, 1H), 7.19-7.29 (m, 3H), 8.03 (m, 1H), 8.03 (m, 1H). FABMS(pos) 264.1 [M+]
References:
EP1382598,2004,A1 Location in patent:Page 39; 78