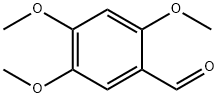
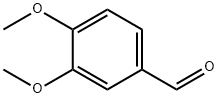
2,4,5-Trimethoxybenzaldehyde synthesis
- Product Name:2,4,5-Trimethoxybenzaldehyde
- CAS Number:4460-86-0
- Molecular formula:C10H12O4
- Molecular Weight:196.2
![[(difluoromethyl)thio]benzene](/CAS/GIF/1535-67-7.gif)
1535-67-7
56 suppliers
$30.00/250mg
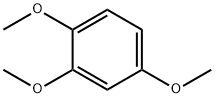
135-77-3
335 suppliers
$5.00/5g

4460-86-0
441 suppliers
$5.00/100mg
Yield:4460-86-0 98%
Reaction Conditions:
Stage #1:difluoromethyl phenyl sulfide;1,2,4-trimethoxy-benzene with tin(IV) chloride in dichloromethane at 20; for 2 h;Inert atmosphere;
Stage #2: with 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione in dichloromethane;water;dimethyl sulfoxide at 20; for 2 h;
Steps:
Aldehydes 4 and 8; General Procedure
General procedure: In a round-bottomed flask equipped with a stirring bar and rubber septum was placed a 1 M solution of SnCl4 in anhydrous CH2Cl2 (1 mL, 1 mmol). To this solution was added PhSCF2H (1; 240.2 mg, 1.5 mmol) in anhydrous CH2Cl2 (1.5 mL), followed by a solution of an aromatic compound (0.5 mmol) in anhydrous CH2Cl2 (1 mL). The reaction was allowed to proceed for 2 h before it was quenched with a solution of IBX (140 mg, 0.5 mmol) in DMSO/H2O (4 mL; 3:1 v:v). After 2 h of stirring at rt, the reaction mixture was quenched by addition of a saturated aqueous solution of sodium thiosulfate (10 mL), then basified with a saturated aqueous solution of sodium hydrogen carbonate (10 mL), followed by stirring and extraction with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with water (3 × 10 mL) and brine (10 mL), dried (anhydrous MgSO4), filtered and concentrated (aspirator). The residue was purified by PTLC, radial chromatography or column chromatography to furnish analytically pure product.
References:
Betterley, Nolan M.;Kongsriprapan, Sopanat;Chaturonrutsamee, Suppisak;Deelertpaiboon, Pramchai;Surawatanawong, Panida;Pohmakotr, Manat;Soorukram, Darunee;Reutrakul, Vichai;Kuhakarn, Chutima [Synthesis,2018,vol. 50,# 10,p. 2033 - 2040]
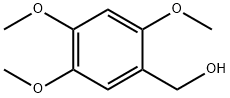
30038-31-4
36 suppliers
$75.00/250mg

4460-86-0
441 suppliers
$5.00/100mg
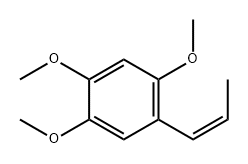
5273-86-9
208 suppliers
$25.00/5mg

4460-86-0
441 suppliers
$5.00/100mg
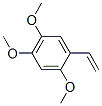
17598-03-7
3 suppliers
inquiry

4460-86-0
441 suppliers
$5.00/100mg

67-56-1
726 suppliers
$7.29/5ml-f
120-14-9
727 suppliers
$5.00/25g

4460-86-0
441 suppliers
$5.00/100mg