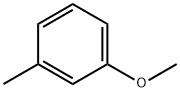
3-Methylanisole synthesis
- Product Name:3-Methylanisole
- CAS Number:100-84-5
- Molecular formula:C8H10O
- Molecular Weight:122.16
Yield:100-84-5 81%
Reaction Conditions:
with dimanganese decacarbonyl at 180; for 1 h;Reagent/catalyst;
Steps:
1-Methoxy-3-methylbenzene (5).
General procedure: General procedure for the alkylation of phenols with dimethyl carbonate. A 17-mL stainless steel high-pressure micro reactor was charged with 3 mmol of Mn2(CO)10, W(CO)6, or Co2(CO)8, 100 mmol of the corresponding phenol, and 300 mmol of dimethyl carbonate, and the reactor was hermetically closed and heated for 1 h at 180°C. The reactor was then cooled to room temperature and opened, and the mixture was filtered through a layer of alumina. Unreacted dimethyl carbonate was distilled off, and the residue was distilled under atmospheric or reduced pressure or recrystallized from ethanol. 1-Methoxy-3-methylbenzene (5). Yield 81%, bp 75.9-76°C (30 mm). 13C NMR spectrum, δC, ppm: 21.76 (CH3), 55.37 (OCH3), 110.72 (C6), 114.68 (C2), 121.44 (C4), 129.18 (C5), 139.47 (C3), 159.52 (C1). Found, %: C 78.49; H 8.21. C8H10O. Calculated, %: C 78.65; H 8.25.
References:
Khusnutdinov;Shchadneva;Mayakova, Yu. Yu. [Russian Journal of Organic Chemistry,2015,vol. 51,# 3,p. 330 - 334][Methylation of Phenol and Its Derivatives with Dimethyl Carbonate in the Presence of Mn2(CO)10, W(CO)6, and Co2(CO)8,2015,vol. 51,# 3,p. 330 - 334,5]
19924-43-7
256 suppliers
$9.00/10g

100-84-5
250 suppliers
$9.00/25g
74-87-3
194 suppliers
$18.50/48622
108-39-4
455 suppliers
$13.00/25g

100-84-5
250 suppliers
$9.00/25g
6971-51-3
282 suppliers
$8.00/5g

100-84-5
250 suppliers
$9.00/25g

