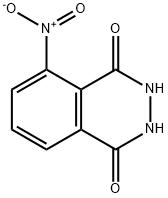
3-Nitrophthalhydrazide synthesis
- Product Name:3-Nitrophthalhydrazide
- CAS Number:3682-15-3
- Molecular formula:C8H5N3O4
- Molecular Weight:207.14
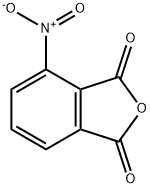
641-70-3
350 suppliers
$6.00/5g

3682-15-3
136 suppliers
$74.00/5g
Yield:3682-15-3 93.7%
Reaction Conditions:
with hydrazine hydrate;acetic acid at 110; for 0.5 h;
Steps:
1
A glass flask of the volume 1 l provided with an agitator, a thermometer, a condenser and a capillary was filled with 600 ml of icy acetic acid and 154.5 g (0.8 mole) of 3-nitrophthalic acid anhydride, thereafter it was heated to the temperature of 110° C. and gradually 44.1 g (0.88 mole) of hydrazine hydrate was added. Next the reaction mixture was kept at the state of boiling for 30 minutes thereafter it was cooled to the temperature of 80° C., the crystals of 5-nitro-2,3-dihydrophthalazine-1,4-dione were filtered, the sediment was washed on a filter with 50 ml of acetic acid and two times with 40 ml of distilled water. The total amount of 5-nitro-2,3-dihydrophthalazine-1,4-dione was 228.7 g, the yield was 92%.Elementary analysis for C8H5N3O4: Found in %: C 46.34; H 2.49; N 20.37.Calculated in %: C 46.38; H 2.44; N 20.29.The filtrate was distilled up to separation of acetic acid, the distillate was mixed and washed with acetic acid, obtained 683 g of acetic acid (93.7%).For regeneration of anhydrous acetic acid, the diluted acetic acid was heated to the temperature 45° C. and gradually 244 g of acetic anhydride was added, after 3 hours of the reaction there was obtained 927 g of regenerated acetic acid anhydride
References:
US2011/275642,2011,A1 Location in patent:Page/Page column 5
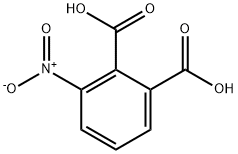
603-11-2
592 suppliers
$15.00/25g

3682-15-3
136 suppliers
$74.00/5g
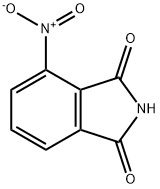
603-62-3
252 suppliers
$9.00/25g

3682-15-3
136 suppliers
$74.00/5g
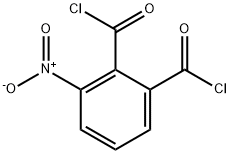
24564-71-4
2 suppliers
inquiry

3682-15-3
136 suppliers
$74.00/5g
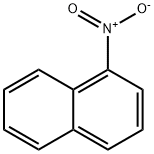
86-57-7
6 suppliers
$14.00/5g

3682-15-3
136 suppliers
$74.00/5g