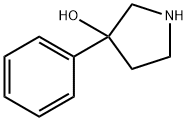
3-phenylpyrrolidin-3-ol synthesis
- Product Name:3-phenylpyrrolidin-3-ol
- CAS Number:49798-31-4
- Molecular formula:C10H13NO
- Molecular Weight:163.22
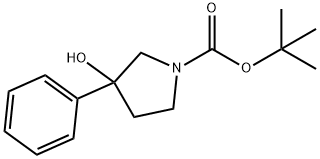
851000-71-0
6 suppliers
inquiry

49798-31-4
24 suppliers
$45.00/10mg
Yield:49798-31-4 240 mg
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 2 h;
Steps:
197.1 Step 1 :
To a solution of tert-butyl 3-oxopyrrolidine-l-carboxylate (1.0 g, 5.4 mmol,1.0 eq) in Et20 (10 mL) at -20 °C under N2, phenylmagnesium bromide (6.4 ml, 6.4 mmol, 1.2 eq) was added. The reaction mixture was stirred at room temperature for 2 hours. The reaction mixture was evaporated to dryness in vacuum. The residue was purified with silica gel chromatography (PE: EA=5: 1) to give the intermediate tert-butyl 3 -hydroxy-3 - phenylpyrrolidine-l-carboxylate (560 mg, 39% yield) as a white solid. Added HC1 in dioxane (2.2 ml, 8.73 mmol, 5.0 eq) to a mixture of tert-butyl 3 -hydroxy-3 -phenylpyrrolidine- 1- carboxylate (460 mg, 1.75 mmol, l.Oeq) in dioxane (3 mL). The reaction mixture was stirred at room temperature for 2 hours. The reaction mixture was evaporated to dryness in vacuum to give 3-phenylpyrrolidin-3-ol (240 mg, 83%) as a pink solid.
References:
WO2020/186220,2020,A1 Location in patent:Paragraph 0395
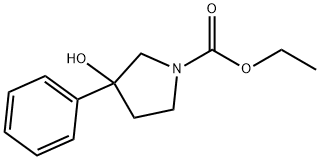
92041-82-2
1 suppliers
inquiry

49798-31-4
24 suppliers
$45.00/10mg
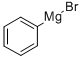
100-58-3
180 suppliers
$18.00/10ml

49798-31-4
24 suppliers
$45.00/10mg