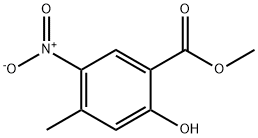
2-Hydroxy-4-Methyl-5-nitro-benzoic acid Methyl ester synthesis
- Product Name:2-Hydroxy-4-Methyl-5-nitro-benzoic acid Methyl ester
- CAS Number:337520-75-9
- Molecular formula:C9H9NO5
- Molecular Weight:211.17
50-85-1
331 suppliers
$10.00/5g

141-78-6
598 suppliers
$22.37/250ml

18107-18-1
211 suppliers
$33.00/5g

337520-75-9
6 suppliers
$65.00/100mg
Yield:337520-75-9 21%
Reaction Conditions:
with nitric acid in methanol;hexane;benzene;
Steps:
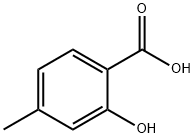
R.128 Methyl 4-Methyl-5-nitrosalicylate
Reference Example 128 Methyl 4-Methyl-5-nitrosalicylate To a solution of 4-methylsalicylic acid (3.5 g) in a mixture of methanol (8 ml) and benzene (32 ml) was added a solution of 2.0M (trimethylsilyl)diazomethane in hexane (15.0 ml) in an ice bath and the mixture was stirred at room temperature for 0.5 hours. The reaction mixture was concentrated in vacuo. To the residual yellow oil was added 69% nitric acid (20 ml) in an ice bath and the mixture was stirred at the same temperature for 2 hours. The reaction mixture was poured into iced Water and extracted with ethyl acetate. The extract was washed with water and brine. The organic layer was dried over anhydrous magnesium sulfate, filtered and the filtrate concentrated in vacuo. The residue was purified by chromatography on a silica gel column using hexane/ethyl acetate=4/1 as an eluant to give the desired compound (1.3 g, yield 21%) as a pale yellow solid. 1H NMR (500 MHz, CDCl3) δ ppm: 2.66 (3H, s), 4.01 (3H, s), 6.92 (1H, s), 8.66 (1H, s).
References:
US6555556,2003,B1

17276-91-4
14 suppliers
inquiry

337520-75-9
6 suppliers
$65.00/100mg