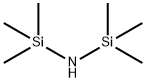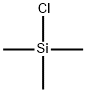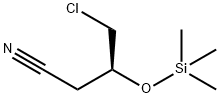
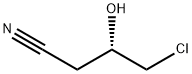
(3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile synthesis
- Product Name:(3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile
- CAS Number:727382-14-1
- Molecular formula:C7H14ClNOSi
- Molecular Weight:191.73
To a solution of (S)-4-chloro-3-hydroxy butanenitrile (52.5 g) in THF (150 mL) was added trimethyl silyl chloride (57.2 g) under inert atmosphere at 25 °C. The resulting reaction mixture was stirred for 10 min at 20°C. A solution of triethyl amine (53.4 g) in THF (100 mL) was added to above reaction mixture by maintaining reaction temperature below 40 °C. To the resulting reaction mixture catalytic amount of sodium iodide was added and stirred further for 5 h at 40°C. Reaction was monitored on GC as well TLC for complete conversion of starting material. After cooling to 0 °C, a 21% aqueous solution of sodium chloride (500 mL) was added and extracted with ethyl acetate (2 X 300 mL). Combined organic layer was dried with anhydrous sodium sulphate, filtered and concentrated under reduced pressure to obtain (3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile as light brown liquid (84.0 g, 99%).
![synthesis of (3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile.png synthesis of (3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile.png](/NewsImg/2022-12-01/6380548217412583178427824.jpg)
Yield:727382-14-1 445.34 g
Reaction Conditions:
in toluene; for 4 h;Reflux;Solvent;
Steps:
1 Synthesis of intermediate (I)
In a 1 L four-necked flask were successively added 150 g of toluene, 300 g of S-4-chloro-3-hydroxybutyronitrile,Stirred at room temperature for 15min until clear, the temperature of the control system is 10 ~ 35 , 306.25g hexamethyldisilazane is added dropwise,The system was heated to the end of the reaction under reflux conditions 4h,To be detected by HPLC S-4-chloro-3-hydroxybutyronitrile residue <0.5%, to stop the reaction;Drying under reduced pressure gave 445.34 g of an oily intermediate in a yield of 103.4% and was used in the next reaction without further purification.
References:
CN105968086,2016,A Location in patent:Paragraph 0081; 0083; 0084; 0085; 0102
7677-24-9
368 suppliers
$19.00/5g

106-89-8
729 suppliers
$9.00/10g
![Butanenitrile, 4-chloro-3-[(trimethylsilyl)oxy]-, (3R)-](/CAS/20210305/GIF/927674-56-4.gif)
927674-56-4
0 suppliers
inquiry
![(3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile](/CAS/20150408/GIF/727382-14-1.gif)
727382-14-1
45 suppliers
inquiry
127913-44-4
276 suppliers
$5.00/5g
4039-32-1
414 suppliers
$15.00/10g
![(3S)-4-Chloro-3-[(triMethylsilyl)oxy]butanenitrile](/CAS/20150408/GIF/727382-14-1.gif)
727382-14-1
45 suppliers
inquiry