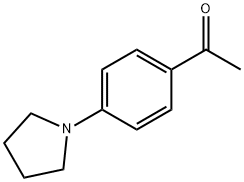
4-(1-PYRROLIDINO)ACETOPHENONE synthesis
- Product Name:4-(1-PYRROLIDINO)ACETOPHENONE
- CAS Number:21557-09-5
- Molecular formula:C12H15NO
- Molecular Weight:189.25
Yield:21557-09-5 99%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 90; for 24 h;Inert atmosphere;
Steps:
General procedure for the preparation of p-aminatedacetophenone (35a-35h)
General procedure: To a solution of compounds 33 (10 mmol), 34a-34h(17 mmol) in dimethyl sulfoxide (25 mL), K2CO3(17 mmol) was added. The reaction was stirred at 90 °Cfor 24 h (35a, 35c, 35e, 35f) or 48 h (35d, 35g, 35h).After that, a saturated aqueous NaCl solution (50 mL) wasadded. Then, the reaction was extracted with ethyl acetate(8 × 25 mL), the organic phase was washed with saturatedaqueous NaCl solution (10 × 50 mL). The aqueous phasewas retro-extracted with ethyl acetate (2 × 25 mL). Organicphases were dried over anhydrous MgSO4 and the solventwas removed under reduced pressure. Compounds 35a, 35c,35e, 35f did not require further purification. Compounds35d, 35g, 35h were purified by column chromatography onsilica gel using hexane/ethyl acetate 8:2 as eluent.1-(4-(Pyrrolidin-1-yl)phenyl)ethanone (34a)Yield: 99%; light brown solid; 1H NMR (300 MHz,CDCl3) d 1.98-2.03 (m, 4H, 2CH2), 2.47 (s, 3H, CH3), 3.33(m, 4H, 2CH2), 6.48 (d, 2H, J 9.0 Hz, Ar-H), 7.83 (d, 2H,J 9.0 Hz, Ar-H); 13C NMR (75 MHz, CDCl3) d 25.4, 25.9,29.7, 110.6, 124.9, 130.7, 150.9, 196.3.
References:
Barbosa, Sandro L.;Baroni, Adriano C. M.;Croda, Júlio;Gomes, Giovana B.;Guerrero, Palimécio G.;Moreira, Flora M. F.;Oliveira, Jefferson R. S.;Perdomo, Renata T.;Shiguemoto, Cristiane Y. K.;das Neves, Amarith R. [Journal of the Brazilian Chemical Society,2020,vol. 31,# 6,p. 1284 - 1295]
![Ethanone, 1-[4-(di-2-propen-1-ylamino)phenyl]-](/CAS/20210305/GIF/1019620-60-0.gif)
1019620-60-0
0 suppliers
inquiry

21557-09-5
55 suppliers
$30.00/5g
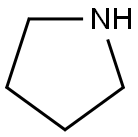
123-75-1
535 suppliers
$10.00/5g
13329-40-3
267 suppliers
$6.00/5g

21557-09-5
55 suppliers
$30.00/5g