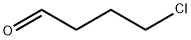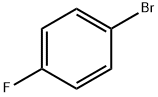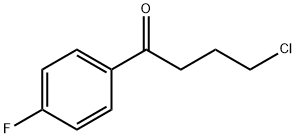
4-Chloro-4'-fluorobutyrophenone synthesis
- Product Name:4-Chloro-4'-fluorobutyrophenone
- CAS Number:3874-54-2
- Molecular formula:C10H10ClFO
- Molecular Weight:200.64
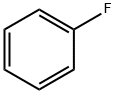
462-06-6
414 suppliers
$10.00/5g
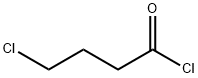
4635-59-0
465 suppliers
$17.67/10gm:

3874-54-2
361 suppliers
$10.00/5G
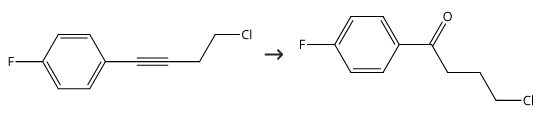
Yield:3874-54-2 90%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 20; for 4 h;Time;
Steps:
1-2
Mix 16 g of fluorobenzene and 20 g of dichloromethane to obtain a fluorobenzene dichloromethane solution (the temperature of the ice bath is guaranteed to be 5°C);After mixing 20g 4-chlorobutyryl chloride and 20g dichloromethane fourth,Obtain a mixed solution of 4-chlorobutyryl chloride and dichloromethane;The mixture of 4-chlorobutyryl chloride and dichloromethane was added dropwise to the fluorobenzene dichloromethane solution at a drip rate of 70 drops/min and mixed with 20 g of anhydrous aluminum chloride, and the second substitution was carried out at 20°C. Reaction for 4h; after the completion of the second substitution reaction, the product of the second substitution reaction is transferred to 600g of ice water for quenching reaction at a speed of 200r/min for 0.3h; the product after the quenching reaction is liquid-separated; The organic phase obtained from the liquid was mixed with 4g of anhydrous sodium sulfate to remove water, then filtered and concentrated (50°C),Obtain 4-chloro-1-(4-fluorophenyl)butan-1-one (with a yield of 90% and a purity of 99%);
References:
CN112538042,2021,A Location in patent:Paragraph 0089; 0095; 0098; 0104
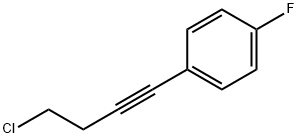
1183278-95-6
2 suppliers
inquiry

3874-54-2
361 suppliers
$10.00/5G
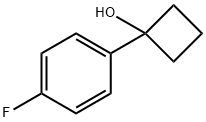
339365-53-6
7 suppliers
inquiry

3874-54-2
361 suppliers
$10.00/5G
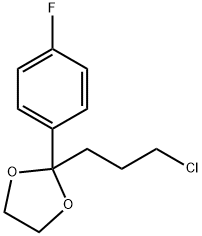
3308-94-9
124 suppliers
$35.00/1g

3874-54-2
361 suppliers
$10.00/5G