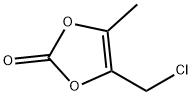
4-Cloromethyl-5-methyl-1,3-dioxol-2-one synthesis
- Product Name:4-Cloromethyl-5-methyl-1,3-dioxol-2-one
- CAS Number:80841-78-7
- Molecular formula:C5H5ClO3
- Molecular Weight:148.54
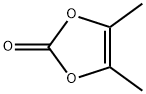
37830-90-3
372 suppliers
$16.00/1g

80841-78-7
536 suppliers
$10.00/250mg
Yield:80841-78-7 89.3%
Reaction Conditions:
with chlorine in 1,2-dichloro-ethane; for 0.5 h;Reflux;Molecular sieve;Solvent;Reagent/catalyst;
Steps:
1-4 Example 2
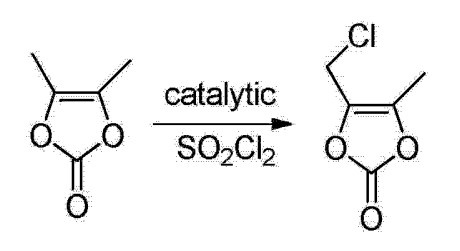
The amount of substance in the chlorine dichloroethane solution is 1.2 times that of 4,5-dimethyl-1,3-dioxol-2-one; the solid base catalyst rare earth molecular sieve Ln(PO2NH)3, the mass is 1% of the mass of 4,5-dimethyl-1,3-dioxol-2-one; the amount of the organic solvent, dichloroethane, is 12 times the mass of 4,5-dimethyl-1,3-dioxol-2-one; the desiccant is anhydrous magnesium sulfate. Add 200mL dichloroethane, 40g (0.35mol) 4,5-dimethyl-1,3-dioxol-2-one, 0.4g solid to a 1000mL four-neck flask equipped with reflux condenser alkali catalyst, heated and stirred until the reaction system refluxed, add 195mL of chlorine dichloroethane solution (0.42mol, 30g of chlorine) dropwise within 30 minutes, keep refluxing for 30 minutes, and check the reaction status by TLC. After completion, the reaction solution is cooled, washed with water, layered, extracted, and the organic phase is dried. Filter to remove the desiccant, the filtrate is at 90, rearrangement under nitrogen protection for 2h, after the rearrangement reaction is over, the reaction solution was distilled under reduced pressure, separated, and purged with nitrogen to obtain crude 4-chloromethyl-5-methyl-1,3-dioxol-2-one; Add 40 mL of ethanol to the above crude product, stir and cool to -5°C, crystallize for 3h, filter with suction, and rinse with ethanol at -5°C. 46.5 g of white crystals were obtained, the yield was 89.3%, and the GC purity was 99.82%.
References:
CN111285837,2020,A Location in patent:Paragraph 0027-0038
95579-71-8
46 suppliers
inquiry

80841-78-7
536 suppliers
$10.00/250mg

37830-90-3
372 suppliers
$16.00/1g

80841-78-7
536 suppliers
$10.00/250mg

95579-71-8
46 suppliers
inquiry
129482-56-0
20 suppliers
inquiry