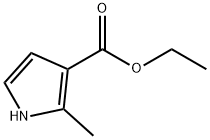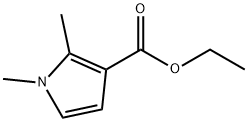
Ethyl 1,2-dimethyl-1H-pyrrole-3-carboxylate synthesis
- Product Name:Ethyl 1,2-dimethyl-1H-pyrrole-3-carboxylate
- CAS Number:19406-11-2
- Molecular formula:C9H13NO2
- Molecular Weight:167.21
Yield:19406-11-2 90%
Reaction Conditions:
Stage #1: 2-methyl-1H-pyrrole-3-carboxylic acid ethyl esterwith sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 0 - 20;
Steps:
Synthesis of compound 3c.
General procedure: To a stirred solution of the substituted 1H-Pyrrole-3-carboxylicacid (765 mg, 5 mmol,) in dry DMF, NaH (300 mg, 7.5 mmol) was added in portions under air at 0 °C. The reaction mixture was then warmed to room temperature and stirred for 30 mins. After cooling to 0 °C again, MeI (1.06 g, 7.5 mmol) was added dropwise. The reaction mixture was warmed to room temperature again and stirred overnight. The reaction mixture was quenched with water, and the aqueous layer was extracted with ether. The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated under vacuum. The residue was purified by flash column chromatography (petroleum ether/ethyl acetate = 5:1) to give compound as colorless solid (751 mg, 90% yield). 3c: 1 H NMR (400 MHz, CDCl 3 ) δ 6.50 (d, J = 2.8 Hz, 1H), 6.46 (d, J = 2.8 Hz, 1H), 4.25 (q, J = 6.8 Hz, 2H), 3.52 (s, 3H), 2.50 (s, 3H), 1.33 (t, J = 6.8 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 165.7, 136.0, 120.9, 112.3, 109.3, 59.3, 33.9, 14.6, 11.0. HRMS ESI (m/z): [M+H] + calcd. for C 9 H 14 NO 2 : 168.1025, found: 168.1024.
References:
Bao, Yan;Wang, Jian-Yong;Zhang, Ya-Xuan;Li, Yan;Wang, Xi-Sheng [Tetrahedron Letters,2018,vol. 59,# 32,p. 3147 - 3150] Location in patent:supporting information
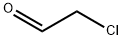
107-20-0
4 suppliers
$22.60/5ml

141-97-9
774 suppliers
$5.00/25g

74-89-5
7 suppliers
$13.44/25ML

19406-11-2
33 suppliers
inquiry
107-20-0
4 suppliers
$22.60/5ml
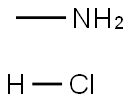
593-51-1
533 suppliers
$21.00/100g

141-97-9
774 suppliers
$5.00/25g

19406-11-2
33 suppliers
inquiry
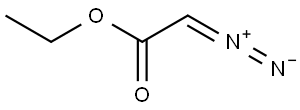
623-73-4
147 suppliers
$27.30/25ML

50874-63-0
0 suppliers
inquiry

19406-11-2
33 suppliers
inquiry
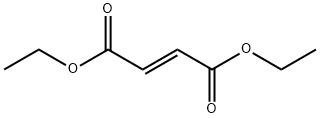
623-91-6
274 suppliers
$6.00/25g
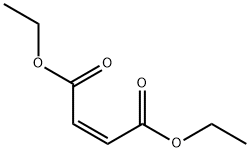
141-05-9
441 suppliers
$6.00/25g