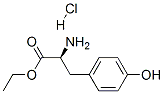
L-Tyrosine Ethyl Ester Hydrochloride synthesis
- Product Name:L-Tyrosine Ethyl Ester Hydrochloride
- CAS Number:4089-07-0
- Molecular formula:C11H16ClNO3
- Molecular Weight:245.7
Yield:4089-07-0 94.7%
Reaction Conditions:
with dichloro sulfoxideReflux;
Steps:
2
Weigh 18g of L-Tyr (tyrosine) and 100ml of ethanol into the reaction flask, add 120ml of SOCl2 (thionyl chloride) dropwise with stirring, and react under reflux. When TLC (Thin Layer Chromatography) detects that there is no L-Tyr (tyrosine) in the reaction system, the reaction is completed, and the reaction solution after the above reaction is vacuum concentrated to a volume of 20 ml, and 100 ml of petroleum ether is added to precipitate a white solid. After centrifugation and drying, 19.8 g of white solid was obtained with a yield of 94.7%.
References:
CN112094204,2020,A Location in patent:Paragraph 0057-0058
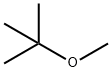
1634-04-4
642 suppliers
$14.00/100g
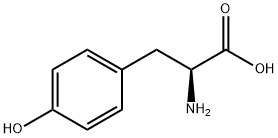
60-18-4
794 suppliers
$15.00/25g

4089-07-0
291 suppliers
$6.00/1g

60-18-4
794 suppliers
$15.00/25g

4089-07-0
291 suppliers
$6.00/1g
