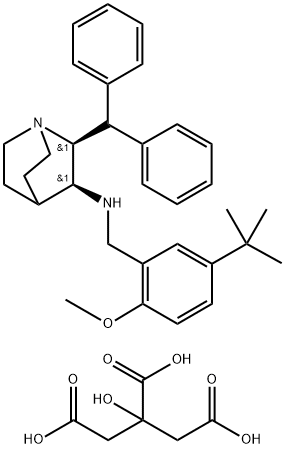
Maropitant citrate hydrate synthesis
- Product Name:Maropitant citrate hydrate
- CAS Number:359875-09-5
- Molecular formula:C38H48N2O8
- Molecular Weight:660.81
147116-67-4
83 suppliers
inquiry
77-92-9
1589 suppliers
$5.00/10g

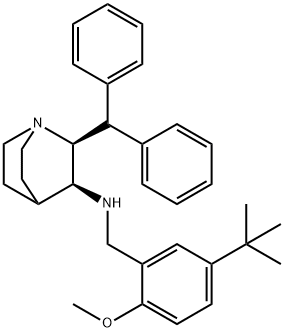
359875-09-5
182 suppliers
$129.00/5amp
Yield:-
Reaction Conditions:
with water in acetone at 38 - 42;
Steps:
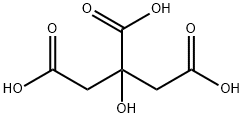
Preparation of (2S, 3S)-2-benzhvdrvl-N- (5-tert-butvl-2-methoxvbenzvl) quinuclidin-3- amine citrate monohydrate, compound of formula la (Step C. Scheme II). A solution of (2S, 3S)-2-benzhydryl-N- (5-tert-butyl-2- methoxybenzyl) quinuclidin-3-amine (33.95 kg, 72.4 moles) and anhydrous citric acid (15.3 kg, 79.7 moles) in a mixture of acetone (215 kg) and water (13.6 kg) was heated to 38-42°C. The resultant mixture was then transferred to another reactor via an in-line filter. The transfer line and filter were washed through with acetone (54 kg) and these filtered washings were added to the solution. The resultant mixture was then cooled to 20-25°C and filtered tert-butyl methyl ether (252kg) was added portion- wise over a period of approximately 35 minutes. The resultant suspension was then granulated at 20-25°C for approximately 20 hours. The solid was then collected by filtration on an agitated filter-dryer and the filter cake was washed twice with filtered tert-butyl methyl ether (50kg each). The resultant solid was then dried at 35°C under vacuum with agitation to give the title compound (44.4kg) as a colourless solid. The product was then milled.'H-NMR (500 MHz, d4-methanol, 30°C) 8 : 7.46 (2H, d), 7.45 (2H, d), 7.37 (4H, m), 7.31 (1 H, m), 7.29 (1 H, m), 7.24 (1 H, dd), 6.95 (1 H, d), 6.76 (1 H, d), 4.75 (1 H, dd), 4.71 (1 H, d), 3.76 (1H, m), 3.57 (1H, d), 3.55 (3H, s), 3.37 (1 H, m), 3.31 (1 H, m), 3.26 (1H, m), 3.24 (1H, d), 3.10 (1H, t), 2.83 (2H, d), 2.75 (2H, d), 2.51 (1H, m), 2.35 (1 H, m), 2.11 (1 H, m), 2.06 (1 H, m), 1.85 (1 H, m), 1.29 (9H, s). 13c NMR (125.7 MHz, d4-methanol, 30°C) 8 : 179.4, 175.0, 156.8, 144.0, 141.5, 141. 4, 131.1, 130.6, 129.4, 128.9, 128. 7, 128. 3, 128.2, 127.2, 126.4, 111.0, 74.0, 64.7, 56.1, 54.2, 50.4, 48.5, 48. 3, 44. 9, 43.8, 34. 8, 32. 9, 25.3, 22.2, 18.1. LRMS (ES+): m/z [MH+1 469.
References:
WO2005/75473,2005,A1 Location in patent:Page/Page column 16; 22-23