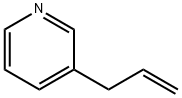
Pyridine, 3-(2-propenyl)- synthesis
- Product Name:Pyridine, 3-(2-propenyl)-
- CAS Number:7300-28-9
- Molecular formula:C8H9N
- Molecular Weight:119.16
Yield:7300-28-9 49.6%
Reaction Conditions:
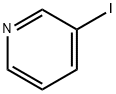
Stage #1: 3-Bromopyridinewith TurboGrignard in tetrahydrofuran at 0; for 6 h;Inert atmosphere;
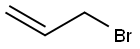
Stage #2: allyl bromide in tetrahydrofuran at 20; for 15 h;Inert atmosphere;
Steps:
4.2.1. 3-Allylpyridine
According to the literature [28], to a 25 mL two-necked round bottomflask, 1.57 g of 3-pyridyl bromide (10 mmol) in dry THF (10 mL)and 15 mL of i-PrMgCl-LiCl were added dropwise under nitrogen at 0 °C.The mixture was stirred for 6 h under 0 °C and 4.76 g of allyl bromide(40 mmol) was added. After the mixture was slowly warmed to room temperature and kept stirring for 15 h, saturated NH4Cl was added andthe resulting mixture was extracted with ethyl acetate. The combinedorganic layers were dried over anhydrous MgSO4 and filtrated, and thesolvent was removed under reduced pressure. The crude product waspurified by column chromatography on silica gel with petroleumether/ethyl acetate (1:1, v/v) as the eluent. The collected yellow oil wasconcentrated to get the product (590 mg, 49.6% yield). 1H NMR (500MHz, CDCl3):δ 8.45 (m, 2H), 7.50 (m, 1H), 7.22 (m, 1H), 5.94 (m, 1H),5.11 (m, 2H), 3.38 (d, J = 5.0 Hz, 2H). 13C NMR (126 MHz, CDCl3):δ 150.11, 147.67, 136.31, 136.24, 135.49, 123.49, 116.96, 37.35.
References:
Zheng, Chaoyue;Huan, Yihong;Tan, Chao;Gao, Deqing [Dyes and Pigments,2021,vol. 188,art. no. 109159]
626-55-1
538 suppliers
$5.00/5/ G

7300-28-9
5 suppliers
inquiry