Applications of Borane-tetrahydrofuran Complex
Nov 13,2019
Borane-tetrahydrofuran complex(BORANE-THF, CAS 14044-65-6) is an important reagent used in the reduction of certain functional groups viz. aldehyde, ketone, carboxylic acid, amide, oxime, imine and nitrile. Borane-tetrahydrofuran complex is also used as hydro borating agent. Borane-tetrahydrofuran complex acts as a borane source for oxazaborolidine catalyzed asymmetric reductions. Borane-Four Tetrahydrofuran complex is a reductive reagent for boron hydride. Borane-Four Tetrahydrofuran complex can be used in the hydrogenation and reduction of the unsaturated bond with certain functional groups, the reduction of the amine reaction in the presence of borane-four Tetrahydrofuran complex, stack Nitride can undergo a transition state of boron hydride to generate amine compounds, while releasing a molecule of nitrogen, the reaction to produce the first-order amine production rate of more than 90%. Borane-Four Tetrahydrofuran complexes can also be used to restore the carbon-nitrogen double bond. In addition, under the action of the Borane-four Tetrahydrofuran complex, the alcohols and aldehydes are generated by nucleophilic addition reaction to produce beta-hydroxy ester, the reaction product as an important intermediate in organic synthesis can be further derived. Borane-Four Tetrahydrofuran complexes are commonly used as organic reductive reagents.
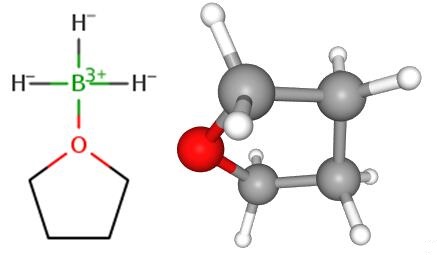
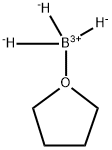
Borane-tetrahydrofuran complex is a dipolar bond charge-transfer complex composed of borane and tetrahydrofuran (THF). These solutions are used for reductions and hydroboration, reactions that are useful in synthesis of organic compounds[1].
Borane-tetrahydrofuran complex is commercially available but can also be generated by the dissolution of diborane in THF. A practical route to this is the oxidation of sodium borohydride with iodine in THF[2].
Borane-tetrahydrofuran complex can reduce carboxylic acids to alcohols and is a common route for the reduction of amino acids to amino alcohols[3] (e.g. valinol). Borane-tetrahydrofuran complex adds across alkenes to give organoboron compounds that are useful intermediates[4]. The following organoboron reagents are prepared from borane-THF: 9-borabicyclo[3.3.1]nonane, Alpine borane, diisopinocampheylborane. Borane-tetrahydrofuran complex is also used as a source of borane (BH3) for the formation of adducts.
Borane-tetrahydrofuran complex reacts with Grignard reagents, arylmercury, arylthalium, and allyl- and propargyllithium compounds to give organoboranes which can be oxidized to the corresponding alcohols, phenols, and 1,3-diols. The reagent is used for blocking tertiary amine groups in Friedel-Crafts cyclizations and oxidative phenol coupling reactions in alkaloid syntheses. Borane-tetrahydrofuran complex activates a,b-unsaturated acids in the reaction with 1,3-dienes and is used in the synthesis of catalysts for asymmetric Diels-Alder and aldol reactions. Trialkylboranes are useful intermediates in the synthesis of compounds labeled with various isotopes for medical applications.
The Borane-tetrahydrofuran complex solution is highly sensitive to air, requiring the use of air-free techniques.
The Borane-tetrahydrofuran complex is stable, but may form explosive peroxides in contact with air. The Borane-tetrahydrofuran complex is incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents, alcohols.The Borane-tetrahydrofuran complex reacts violently with water. The Borane-tetrahydrofuran complex is highly flammable, and should be stored under inert gas.
References
[1] Marek Zaidlewicz, Herbert C. Brown, Santhosh F. Neelamkavil, "Borane–Tetrahydrofuran" Encyclopedia of Reagents for Organic Synthesis, 2008 John Wiley & Sons.
[2] Kanth, J. V. Bhaskar; Periasamy, Mariappan (1 September 1991). "Selective reduction of carboxylic acids into alcohols using sodium borohydride and iodine". The Journal of Organic Chemistry. 56 (20): 5964–5965.
[3] McKennon, Marc J.; Meyers, A. I.; Drauz, Karlheinz; Schwarm, Michael (June 1993). "A convenient reduction of amino acids and their derivatives". The Journal of Organic Chemistry. 58 (13): 3568–3571.
[4] Kabalka, George W.; Maddox, John T.; Shoup, Timothy; Bowers, Karla R. (1996). "A Simple And Convenient Method For The Oxidation Of Organoboranes Using Sodium Perborate: (+)-isopinocampheol". Organic Syntheses. 73: 116.
- Related articles
- Related Qustion
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol.....
Nov 13,2019Chemical ReagentsMethyl salicylate is an external analgesic available in over-the-counter (OTC) medicines that temporarily relieve minor body aches and muscle and joint pain associated with backache, arthritis, strains, sprains, and bruises.....
Nov 13,2019Flavors and fragrancesBorane-tetrahydrofuran complex
14044-65-6You may like
Borane-tetrahydrofuran complex manufacturers
- Borane-tetrahydrofuran complex
-

- $5.00 / 200kg
- 2025-12-15
- CAS:14044-65-6
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 200mt/year
- Borane-tetrahydrofuran complex
-

- $0.00 / 1KG
- 2025-06-27
- CAS:14044-65-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Borane-tetrahydrofuran complex
-

- $9.00 / 1KG
- 2025-05-26
- CAS:14044-65-6
- Min. Order: 1KG
- Purity: 99.8%
- Supply Ability: 100tons