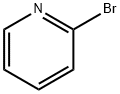
2-Bromopyridine: Versatile Building Block for Heterocycle Synthesis
Dec 10,2025
2-Bromopyridine is an organic compound that is widely used as a building block in organic synthesis. It is also used as intermediate for the synthesis of Pyridine derivatives. 2-Bromopyridine can be used as: a building block in the formation of C−N bond by various cross coupling reactions; a reactant in Negishi cross-coupling reaction with aryl halides catalyzed by palladium; a reactant in the synthesis of 2′-pyridyldifluoroacetate with ethyl bromodifluoroacetate in presence of copper as a catalyst.

Synthesis of MAPA Reagents from 2-Bromopyridine
The 2-methylaminopyridine formamide and related amides (MAPA) have been widely used as reagents for formylation and acylation of various organometallic reagents, amines, and other nucleophiles. A potentially inexpensive route to many MAPA-type compounds would be the direct conversion of 2-bromopyridine to the corresponding N-methyl amides via the Goldberg reaction. Although the catalytic Goldberg reaction has been extensively studied in recent years using various aryl and heteroaryl halides, only a few examples have been reported that provide MAPA-related compounds from 2-halopyridines. A practical large-scale conversion of 2-bromopyridine to MAPA reagents would require inexpensive ligands, copper salts, reagents, and solvents. In addition, low catalyst loading, low reagent and solvent toxicity, reasonable reaction temperature, and a simple workup would be desirable. Although many ligands have been found to promote the catalytic Goldberg reaction, diamine ligands appeared to be good candidates for the proposed study.[1]
A general and economical method for the synthesis of various 2-methylaminopyridine amides (MAPA) from 2-bromopyridine has been developed. The catalytic Goldberg reaction using copper iodide and 1,10-phenanthroline as a catalyst converts 2-bromopyridine and secondary N-methylamides into the desired MAPA compounds in excellent yields. Although DMEDA works well as the ligand in some of the reactions studied, phen is more effective when a sterically hindered or less reactive secondary amide is the coupling partner. A modification of this process using an N-alkyl(aryl)formamide as a starting material provides a one-pot synthesis of 2-alkyl(aryl)aminopyridines from 2-bromopyridine. The intermediate aminopyridine formamide is cleaved via in situ methanolysis or hydrolysis to afford the N-substituted 2-aminopyridine products in high yields. Both of these methods can conveniently be carried out on a multi-gram scale.
2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones
Pyridine derivatives and related azaheterocycles are widely found as a central structural motif in biologically active compounds, natural products, and functional materials. Recently we attempted the synthesis of hexaheteroarylbenzenes by applying Ru(II)-catalyzed multiple C–H functionalization of benzene substrates with pyridyl, pyrimidyl, or pyrazolyl directing groups. Interestingly, in the case of 2-pyridylbenzene as the substrate and 3-methyl-2-bromopyridine (1a) as the arylating agent, di-ortho-heteroarylated 2-pyridylbenzene P was isolated as the major product together with 2-pyridone by-products. To our best knowledge, there is only one report of the direct conversion of 2-halopyridines to N-(2-pyridyl)pyridin-2-ones, which was achieved by using a CuI-trans-N,N’-dimethylcyclohexane-1,2-diamine-K2CO3 catalyst system. The authors hypothesized that 2-bromopyridine hydrolyzes under the established reaction conditions and undergoes a coupling reaction with non-hydrolyzed 2-bromopyridine, but the reaction pathway was not examined.[2]
In summary, we have developed a novel synthetic route to polyheteroarylated 2-pyridones from various substituted 2-bromopyridines. As a result, 2-pyridones with different degrees of 2-pyridyl substitution and, in particular, pyridone with one (2-pyridyl) C–N bond and with 2-, 4- ,5-, and 6-(2-pyridyl) C–C bonds, can now be readily prepared. The small scope of the various 2-bromopyridine starting compounds indicates that electron-withdrawing groups have an advantage over electron-donating groups in pyridone formation. The preliminary mechanistic study suggests that the oxygen required for 2-pyridone formation is derived from carbonate. Although the yields of 2-pyridone products were only moderate, the catalytic method represents an interesting synthetic route to construct complex molecular scaffolds from simple precursors. The prepared 2-pyridone derivatives can be used as ligands in coordination with transition metals for supramolecular architectures or for the preparation of multimetallic catalytically active species.
References
[1]Comins DL. Synthesis of MAPA Reagents and 2-Alkyl(aryl)aminopyridines from 2-Bromopyridine Using the Goldberg Reaction. Molecules. 2022 Mar 11;27(6):1833. doi: 10.3390/molecules27061833. PMID: 35335206; PMCID: PMC8952803.
[2]Drev M, Brodnik H, Grošelj U, Perdih F, Svete J, Štefane B, Požgan F. 2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones via Ru(II)-Mediated Domino C-O/C-N/C-C Bond Formation Reactions. Molecules. 2024 Sep 17;29(18):4418. doi: 10.3390/molecules29184418. PMID: 39339413; PMCID: PMC11433726.
- Related articles
- Related Qustion
Ribociclib is used in combination with another medication to treat a certain type of hormone receptor–positive (depends on hormones such as estrogen to grow) advanced breast cancer.....
Dec 10,2025Biochemical EngineeringFerrous oxalate dihydrate serves as photo-Fenton catalyst, undergoes polymorph-dependent thermal dehydration, and is tunable via crystallization additives.....
Dec 10,2025Chemical Materials2-Bromopyridine
109-04-6You may like
- 2-Bromopyridine
-

- $10.00 / 1KG
- 2025-12-11
- CAS:109-04-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5tons
- 2-Bromopyridine
-

- $0.00 / 1KG
- 2025-12-11
- CAS:109-04-6
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10ton/month
- 2-Bromopyridine
-
- $0.00 / 25KG
- 2025-12-01
- CAS:109-04-6
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 10000KGS