Applications of Potassium pyrophosphate
Jan 6,2020
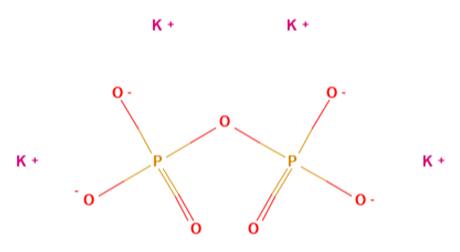
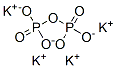
Fig 1. Chemical structure formula and three-dimensional structure of Potassium pyrophosphate
Potassium pyrophosphate is a whitish-colored powdered solid. Potassium pyrophosphate is toxic by ingestion, and/or inhalation. Contact with the substance should be avoided. Potassium pyrophosphate is mainly used as an emulsifier, quality improver and metal ion chelating agent. Potassium pyrophosphate can be found in herbicidal compositions as a spray adjuvant. Potassium pyrophosphate is also used as an anionic dispersant in water-based (latex) paints[1].
Potassium pyrophosphate is in the form of colourless or white crystals, or a white crystalline or granular powder. Potassium pyrophosphate is a hydroscopic solid, soluble in water, insoluble in ethanol. Potassium pyrophosphate has a higher solubility in water treatment formulations than sodium derivatives.
Potassium pyrophosphate is mainly used as an emulsifier, quality improver, metal ion chelating agent. Potassium pyrophosphate keeps the freshness, holds water and improves the quality of quick-frozen marine products. Potassium pyrophosphate can be applied to prevent the struvite of canned seafoods and the discoloration of fruit products, to improve ice cream swelling rate or the taste of noodle and cake, and prevent the cheese aging. Potassium pyrophosphate is used as a pH buffer and as a dough conditioner in soy-based "meat alternatives", chicken nuggets and imitation crab and lobster products. Potassium pyrophosphate is a thickening agent in instant puddings[2].
Potassium pyrophosphate can be found in herbicidal compositions as a spray adjuvant That adjuvant serves as both a plant nutrient and surface-active agent and functions synergistically with the herbicide to increase its level of absorption into plant tissues and to stimulate both the target and non-target plant species to growth responses for increased herbicide efficacy.
Potassium pyrophosphate is classifed as a buffering, chelating and oral care agent. Potassium pyrophosphate is the "tartar control" agent which removes calcium and magnesium from the saliva, so they can not deposit on the teeth. Potassium pyrophosphate is mainly used in toothpastes and mouthwash formulations[3].
Potassium pyrophosphate is used as an anionic dispersant in water-based (latex) paints.It removes a small amount of Fe3+ in order to improve the quality of dyeing.
Potassium pyrophosphate is a soap and detergent builder. Potassium pyrophosphate is a water softener and an emulsifier to suspend oils and prevent them from redepositing on clothing in the wash. As a water softener, it combines with magnesium to sequester it from the detergent, without precipitating it onto the clothing. As a detergent additive, it can also "reactivate" detergents or soaps that have combined with calcium to make an insoluble scum. Because of the eutrophication of water it is seldom used as a detergent additive[4].
References
[1] R. P. Buc.; Samang. Singhadeja.; L. B. Rogers. Ultraviolet Absorption Spectra of Some Inorganic Ions in Aqueous Solutions. Anal. Chem. 1954, 26 (7),1240-1242.
[2] C. L. BASCOMB, & K. THANIGASALAM. . Comparison of aqueous acetylacetone and potassium pyrophosphate solutions for selective extraction of organic-bound fe from soils. European Journal of Soil Science, 29(3), 382-387.
[3] Sobel, A. E., & Kramer, B. (1933). A new colorimetric method for the quantitative estimation of small amounts of potassium. , 100(2), 561-571.
[4] Jin-Young CHO, Duck-Young HWANG, Dong-H eon LEE, Bongyoung YOO, & Dong-Hyuk SHIN. . Influence of potassium pyrophosphate in electrolyte on coated layer of az91 mg alloy formed by plasma electrolytic oxidation. Transactions of Nonferrous Metals Society of China(4), 44-48.
[5] https://www.chemspider.com/Chemical-Structure.22198.html?rid=6be954f3-6560-4f71-a658-7cb4b91887ad&page_num=0
[6] https://pubchem.ncbi.nlm.nih.gov/compound/23740
- Related articles
- Related Qustion
- Potassium pyrophosphate: Applications and Uses Apr 7, 2023
Potassium pyrophosphate (K4P2O7) is a water soluble, buffering and chelating agent.
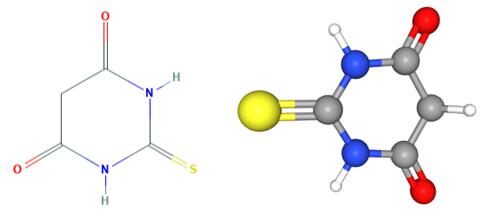
2-thiobarbituric acid is a barbiturate, the structure of which is that of barbituric acid in which the oxygen at C-2 is replaced by sulfur. It has a role as a reagent and an allergen.....
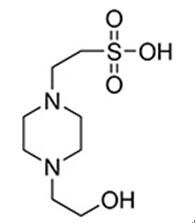
Jan 6,2020Chemical Reagents4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid (HEPES) has been described as one of the best all-purpose buffers available for use in biological research.....
Jan 6,2020Chemical ReagentsPotassium pyrophosphate
7320-34-5You may like
- Dimolybdenum pentaboride Crystal
Apr 25, 2024
- The structure of Molybdenum silicide
Apr 25, 2024
- What is the crystal structure of Uranium boride?
Apr 25, 2024
Potassium pyrophosphate manufacturers
- Potassium Diphosphate
-

- $30.00 / 1kg
- 2024-04-26
- CAS:7320-34-5
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 5000
- Potassium pyrophosphate
-

- $6.00 / 1KG
- 2024-04-12
- CAS:7320-34-5
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 500KG/Month
- Potassium Diphosphate
-

- $1.00 / 1000KG
- 2024-04-08
- CAS:7320-34-5
- Min. Order: 1000KG
- Purity: 98%
- Supply Ability: 20tons per month