What is 4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid( HEPES)?
Jan 6,2020
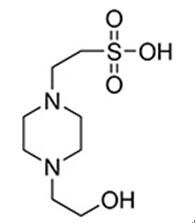
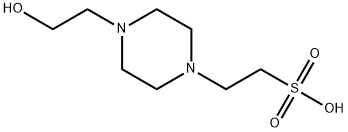
Fig 1. Chemical structure formula and three-dimensional structure of 4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid
4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid (HEPES) has been described as one of the best all-purpose buffers available for use in biological research. The molecule is zwitterionic at most biological pHs and is most effective as a buffer at pH 6.0 to 8.5. May be used in isoelectric focusing applications and as a buffer in the quantitative and selective measurement of antigen-antibody reactions. HEPES is reportedly superior to NaHCO3 in controlling pH in tissue and organ culture. Not recommended for use with the Folin-Ciocalteu protein assay[1-3].
A buffer solution of 4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid can be prepared by any of several methods. The free acid can be added to water, then titrated with sodium hydroxide or potassium hydroxide to the pH desired. Alternatively, equimolar concentrations of HEPES and of sodium HEPES (sc-216092) can be mixed in approximately equal volumes, back-titrating with either solution to the appropriate pH.
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid is a zwitterionic sulfonic acid buffering agent; one of the twenty Good's buffers. It is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration (produced by aerobic respiration) when compared to bicarbonate buffers, which are also commonly used in cell culture. The dissociation of water decreases with falling temperature, but the dissociation constants (pK) of many other buffers do not change much with temperature. It is like water in that its dissociation decreases as the temperature decreases. This makes 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid a more effective buffering agent for maintaining enzyme structure and function at low temperatures. Lepe-Zuniga et al. reported an unwanted photochemical process wherein 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid when exposed to ambient light produces hydrogen peroxide, which is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-containing solutions in darkness as much as possible to prevent oxidation[4-6].
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid 1M is used as a buffer alternative from sodium bicarbonate for cell culture. 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid is effective as a buffer at pH 6.8 to 8.2. 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid is also attributed with characteristics such as: pKa values between 6.0 and 8.0, high solubility membrane impermeability, limited effect on biochemical reactions, very low visible and ultraviolet light absorbance, chemically and enzymatically stable, and easy to prepare.
It is widely used in many biochemical reactions and as a buffering agent in some cell culture media. It is reportedly superior to NaHCO3 in controlling pH in tissue and organ culture. It is commonly used as a strong buffering agent.
References
[1] Wilfred J. Ferguson, K I Braunschweiger, W R Braunschweiger. Hydrogen ion buffers for biological research[J]. Analytical Biochemistry, 1980, 104(2):300-310.
[2] Medzon, Edward L, Gedies, Adolph. Substitution of 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES) for bicarbonate in protein-free animal cell culture medium: application to vaccinia virus quantitation and fluorogenic acetylesterase assay in living LM cells[J]. Canadian Journal of Microbiology, 17(5):651-653.
[3] Himmel H M, Heller W. Studies on the interference of selected substances with two modifications of the Lowry protein determination[J]. 1987, 25(12):909-913.
[4] Baicu SC, Taylor MJ (2002). "Acid-base buffering in organ preservation solutions as a function of temperature: new parameters for comparing buffer capacity and efficiency". Cryobiology. 45 (1): 33–48.
[5] https://pubchem.ncbi.nlm.nih.gov/compound/23831
[6] https://www.chemspider.com/Chemical-Structure.22278.html?rid=d52c8547-0451-4ce5-96e0-fea4141965e6
- Related articles
- Related Qustion
- HEPES: Chemical Properties and Applications in Cell Culture Oct 30, 2024
HEPES, a zwitterionic buffer, maintains pH stability, influences cellular functions, and inhibits prion protein conversion, making it essential in biological and medical research.
- Uses and characteristics of HEPES buffer Apr 13, 2022
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a zwitterionic sulfonic acid buffering agent; one of the twenty Good's buffers.
Potassium pyrophosphate is a whitish-colored powdered solid. Potassium pyrophosphate is toxic by ingestion, and/or inhalation. Contact with the substance should be avoided.....
Jan 6,2020Inorganic saltsCommonly known as Baby Foam due to its exceptional mildness, Sodium Cocoyl Isethionate Raw Material is a surfactant that is comprised of a type of sulphonic acid called Isethionic Acid.....
Jan 6,2020Organic reagentsHEPES
7365-45-9You may like
- HEPES
-

- $0.00 / 1G/KG
- 2025-12-19
- CAS:7365-45-9
- Min. Order: 1G/KG
- Purity: 99%
- Supply Ability: 100000000KG
- 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
-

- $0.00 / 25Kg/Drum
- 2025-12-18
- CAS:7365-45-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt
- HEPES
-

- $41.00 / 500mg
- 2025-12-17
- CAS:7365-45-9
- Min. Order:
- Purity: 99.82%
- Supply Ability: 10g