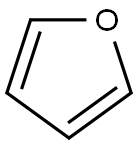
Electrophilic Reactions of Furan
Jan 24,2022
Furan is fairly soluble in organic solvents, including alcohol and ether, but slightly soluble in water. They are toxic and may be carcinogenic in humans. The parent furan has a bp of 31.5°C. The ionization potential is 8.89 eV and the dipole moment is 0.71 D with the negative end on the oxygen atom. The UV/visible absorption maximum of parent furan in ethanol is λmax 208 nm (ε 3.99). The chemical shifts of parent furan in the 1H NMR spectrum showed two doublets at δ 7.29 due to C2–H and C5–H protons because both are present in identical environments, while other doublets in highfield at δ 6.24 are due to C3–H and C4–H protons. The chemical shifts in the 13C NMR spectrum showed two peaks at δ 143.6 ppm for C2 and C5 and at δ 110.4 ppm for C3 and C4 carbon atoms.
Chemical Reactivity
The computed resonance energy of furan in a quantitative manner is 18 kcal/mol, while for thiophene it is 29 kcal/mol. The lower resonance energy of furan makes it less stable than thiophene.
Electrophilic Reactions
Because furan is a π-rich heterocycle, electron density on the ring is higher compared to normal aromatic compounds and thereby electrophilic reactions in furan are 6 × 1011 times faster than benzene. The electrophilic substitution reactions in furan proceed via addition–elimination reactions. The attachment of an electrophile at the C2 and C3 sites creates a positive charge on the ring and is delocalized over the ring atoms. The cation formed by addition of an electrophile at C2 has three possible resonating structures responsible for higher stability, while the cation formed by attachment at C3 has only two resonating structures, which makes it less stable and reactive. Thus the preferential site for electrophilic substitution is C2 and then C5.

Additionally, the distribution of π-electron density at oxygen and the carbon atoms varied considerably. The overall differences are manifested in their calculated π-electron density.
- Related articles
- Related Qustion
- Uses of Furan Jan 24, 2022
Furan is a five-membered aromatic oxygen heterocycle, comprised of four conjugated carbon atoms and one oxygen atom in a cyclic system. Furan is a planar aromatic compound because one of the two lone pairs of electrons on the oxygen partici
- Toxicity and hazards of Furan Oct 27, 2021
Furan can cause eye, skin, and mucus membrane irritation, burning sensation and, in severe cases, corrosion. If inhaled, furan may produce pulmonary edema and bronchiolar necrosis.
Benzoxazole is an important class of heterocycle having a bicyclic structure in which an aryl ring is fused with the “d” site of an oxazole moiety.....
Jan 24,2022Organic SolventsIsothiazole (1,2-thiazoles) is a five-membered heteroaromatic and considered to be derived from thiophene in which the second position is occupied by a nitrogen atom.....
Jan 24,2022APIFuran
110-00-9You may like
- Furan
-
- $1.10 / 1g
- 2023-07-27
- CAS:110-00-9
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
- Furan
-

- $200.00 / 1kg
- 2023-06-26
- CAS:110-00-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
- Furan
-

- $200.00 / 1kg
- 2023-05-27
- CAS:110-00-9
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 50000 tons