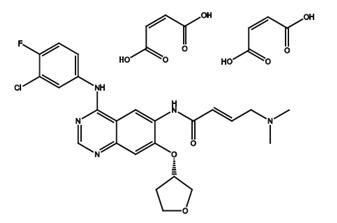
Lenvatinib - pharmacodynamics
Dec 13,2019
Lenvatinib (LenvimaTM) is a multitargeted receptor kinase inhibitor that inhibits the kinase activities of vascular endothelial-derived growth factor receptors 1, 2 and 3, fibroblast growth factor receptors 1, 2, 3 and 4, platelet-derived growth factor receptor ɑ, RET and KIT. In addition to their role in normal cellular function, these kinases have been implicated in pathogenic angiogenesis, tumour growth and cancer progression[1].
Based on x-ray crystallography and kinetic interaction studies, Lenvatinib binds to the adenosine 50-triphosphate binding site of VEGFR2 and to a neighbouring region via a cyclopropane ring and thereby inhibits tyrosine kinase activity and associated signalling pathways [2].
In preclinical in vitro studies and in vivo studies, Lenvatinib exhibited potent antitumour activity via inhibition of tyrosine kinase activities of VEGFR 1–3 and other pro-angiogenic and oncogenic pathway-related RTKs. In an in vitro assay of angiogenesis, Lenvatinib inhibited VEGFR- and FGFR-induced proliferation and tube formation of human umbilical vein endothelial cells. Lenvatinib treatment also inhibited angiogenesis and FGFR and RET signaling pathways in human differentiated, anaplastic and medullary thyroid cancer xenograft mouse models. In cultured thyroid cancer cell lines, Lenvatinib inhibited cell proliferation in 2 of 11 lines, and inhibited phosphorylation of FGFR1 and its downstream effector FGFR substrate 2 (FRS2). Lenvatinib (typically at concentrations of 30–100 nmol/L) inhibited pro-oncogenic RET gene fusion signalling in human thyroid and lung cancer cell lines, including inhibition of anchorage dependent and -independent growth and oncogenic activity of these RET gene transformed cell lines. Lenvatinib also suppressed tumour growth and significantly decreased microvessel density in mouse RET gene fusion driven tumour models. In ex vivo analyses, Lenvatinib treatment reduced phosphorylation of KIFBRET and MAPK (also known as extracellular signalregulated kinases; ERK) in mice with NIH3T3/KIF5BRET tumours. Lenvatinib also reduced microvessel density and inhibited angiogenesis in human sarcoma xenografts resistant to at least one or more clinically relevant reference drugs (doxorubicin, cisplatin or ifosfamide given at the maximum tolerated dose). Since Lenvatinib inhibits both VEGFRs and FGFRs, the drug may provide a mechanism of overcoming resistance to drugs that only inhibit VEGF/VEGFR[3-4].
In phase 1 and 2 studies, oral Lenvatinib exhibited antitumour activity against a variety of tumour types, including unresectable advanced medullary thyroid cancer and advanced hepatocellular carcinoma. In phase 1 dose-escalation study in patients with advanced solid tumours, oral Lenvatinib was associated with tumour shrinkage and changes in plasma angiogenic proteins (potential biomarkers for Lenvatinib-inducedantitumour activity), including increasing plasma VEGF and stromal cell-derived factor 1a (SDF1a) levels and decreasing plasma levels of soluble VEGFR2. These changes occurred in a dose-dependent manner, with maximum tumour shrinkage correlated with increases in plasma SDF1a levels[5].
Lenvatinib is being developed by Eisai Co. Ltd as an anticancer agent and was approved by the US FDA in February 2015 for locally recurrent or metastatic, progressive, RAI-refractory differentiated thyroid cancer in the USA at an oral dosage of 24 mg once daily. At present, an ongoing global, phase 3 trial is evaluating the use of Lenvatinib as first-line treatment in unresectable hepatocellular carcinoma, and global phase 2 and phase 1/2 trials are evaluating its use for several other malignancies, including melanoma, renal cell carcinoma and non-small cell lung cancer (NSCLC) [6].
References
1.Stjepanovic N, Capdevila J. Multikinase inhibitors in the treatment of thyroid cancer: specific role of Lenvatinib[J]. Biologics. 2014, 8:129–39.
2.Okamoto K, Ikemori-Kawada M, Jestel A. Distinct binding mode of multikinase inhibitor Lenvatinib revealed by biochemical characterization[J]. ACS Med Chem Lett. 2015, 6(1):89–94.
3.Yamamoto Y, Matsui J, Matsushima T. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage[J]. Vasc Cell. 2014; 6:18
4.Okamoto K, Kodama K, Takase K. Antitumor activities of the targeted multi-tyrosine kinase inhibitor Lenvatinib (E7080) against RET gene fusion-driven tumor models[J]. Cancer Lett. 2013, 340(1):97–103
5.Molina AM, Hutson TE, Larkin J. A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor Lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC)[J]. Cancer Chemother Pharmacol. 2014, 73 (1):181–9.
6.Shumaker R, Aluri J, Fan J. Evaluation of the effects of formulation and food on the pharmacokinetics of Lenvatinib (E7080) in healthy volunteers[J]. Int J Clin Pharmacol Ther. 2014, 52(4):284–91
- Related articles
- Related Qustion
- Lenvatinib: Uses, Dose, Mechanism of action and Side effects Apr 3, 2024
Lenvatinib is a prescription anticancer drug that belongs to the class of multi-receptor tyrosine kinase inhibitors.
- Lenvatinib: mechanism of action and anti-cancer therapy Jul 5, 2023
Lenvatinib is a potent inhibitor of the VEGF/VEGFR signaling pathway, which plays a crucial role in tumor angiogenesis.
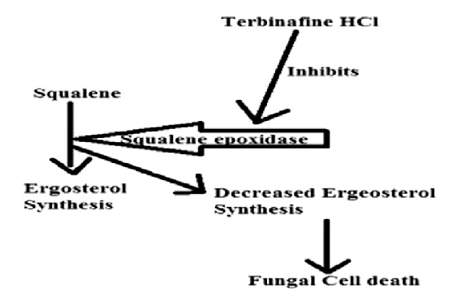
Terbinafine hydrochloride is a synthetic allylamine antifungal with a broad spectrum and used to treat dermatophyte infections of the toenail/fingernail, ringworm and jock itch[1].....
Dec 12,2019APIAfatinib dimaleate (Tovok; BIBW2992; Gilotrif) is a salt form of Afatinib. Afatinib is a second-generation, orally administered, irreversible inhibitor of the ErbB family of tyrosine kinases.....
Dec 13,2019InhibitorsLenvatinib
417716-92-8You may like
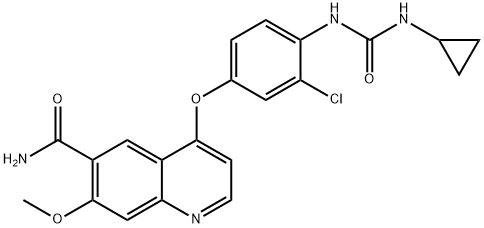
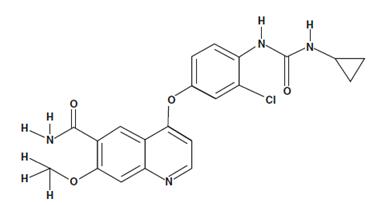
- 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide
-
![417716-92-8 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide](/ProductImageEN1/2024-11/Small/dcc7e230-3cd9-4044-bd40-55f71aa4459e.gif)
- $0.00 / 10g
- 2025-07-11
- CAS:417716-92-8
- Min. Order: 10g
- Purity: 99% HPLC
- Supply Ability: 1000kg
- 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide
-
![417716-92-8 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide](/ProductImageEN/2023-09/Small/bdeaee7d-2440-4c27-9c73-4af9c0d1f608.png)
- $0.00 / 1KG
- 2025-07-11
- CAS:417716-92-8
- Min. Order: 1KG
- Purity: 99.9% HPLC
- Supply Ability: 1000
- Lenvatinib
-

- $5.00/ KG
- 2025-07-11
- CAS:417716-92-8
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS