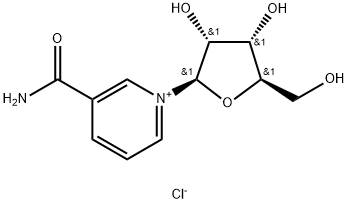
Safety of taking nicotinamide nucleoside chloride
Aug 19,2019
Abstract
Nicotinamide riboside (NR) is a newly discovered nicotinamide adenine dinucleotide (NAD+) precursor vitamin. A crystal form of NR chloride termed NIAGEN is generally recognized as safe (GRAS) for use in foods and the subject of two New Dietary Ingredient Notifications for use in dietary supplements. To evaluate the kinetics and dose-dependency of NR oral availability and safety in overweight, but otherwise healthy men and women, an 8-week randomized, double-blind, placebo-controlled clinical trial was conducted. Consumption of 100, 300 and 1000 mg NR dose-dependently and significantly increased whole blood NAD+ (i.e., 22%, 51% and 142%) and other NAD+ metabolites within 2 weeks. The increases were maintained throughout the remainder of the study. There were no reports of flushing and no significant differences in adverse events between the NR and placebo-treated groups or between groups at different NR doses. NR also did not elevate low density lipoprotein cholesterol or dysregulate 1-carbon metabolism. Together these data support the development of a tolerable upper intake limit for NR based on human data.
Introduction
The NAD+ co-enzymes NAD+, NADH, NADP+ and NADPH are the central regulators of metabolism. They are required for fuel oxidation, ATP generation, gluconeogenesis, ketogenesis, production of pentose phosphates, heme, lipids, steroid hormones and detoxification of free radical species1,2. NAD+ is also a consumed substrate of enzymes that polymerize and/or transfer ADPribose, form cyclic ADPribose (cyclic ADPribose synthetases) and deacylate protein lysine substrates (sirtuins) with production of acyl-ADPribosyl products. Poly(ADPribose) polymerases (PARPs) signal DNA damage in order to assemble repair machinery, while cyclic ADPribose synthetases produce second messengers that mobilize calcium ions from intracellular stores, and sirtuins influence gene expression and protein activities by virtue of reversing protein post-translational modifications3. In light of the important roles of NAD+ co-enzymes in metabolism and mediating some of the longevity benefits of calorie restriction via sirtuins, there is a renewed interest in the synthesis and maintenance of the NAD+ metabolome4.
All tissues produce NAD+ from nicotinamide (NAM) or the recently identified NAD+ precursor, nicotinamide riboside (NR)5 Some tissues can produce NAD+ from tryptophan de novo and nicotinic acid (NA)2, although the generation of NAD+ from tryptophan is much less efficient than from the vitamin precursors of NA, NAM, or NR, which are collectively termed vitamin B3. NAD+ can also be supported by dietary precursors6. For example, pellagra, a disease of deficiency of NAD+ precursors, can be prevented or treated with approximately 15 mg/day of NA or NAM or with 60-times as much tryptophan7. Importantly, despite homeostatic systems and dietary intake of NAD+ precursors, it is now known that the levels of NAD+ co-enzymes are continuously challenged by metabolic stress. In the overfed and type 2 diabetic mouse livers, levels of NADPH are strikingly depressed8, whereas in noise-induced hearing loss9, heart failure10, peripheral nerve damage11, central brain injury12 and the liver of a lactating mouse13, NAD+ levels are compromised. Moreover, NAD+ levels have been reported to decline in response to DNA damage14, alcohol metabolism15, and aging16,17, and the expression of nicotinamide phosphoribosyltransferase (NAMPT), the enzyme required for NAM salvage, declines with aging18 and chronic inflammation19. Thus, considering the relationships between NAD+, metabolic stress and aging, nutritional scientists are now investigating whether the ingestion of higher levels of a B3 vitamin should be part of an evidence-based approach to optimize health2.
Although NA, NAM, and NR all produce NAD+ and NADP+2,7,20, it is important to note that each precursor has unique effects physiologically. NA can lower blood lipids and is used to treat dyslipidemia21. At doses of greater than 50 mg/day, NA can also induce flushing6,21. In contrast, NAM does not lower blood lipids or cause flushing, has been reported be a sirtuin inhibitor at high doses20,22, and appears to have a greater effect at elevating blood levels of homocysteine (HCY) in humans than NA via its metabolism to 1-methylnicotinamide (MeNAM)23. In yeast, NR activates SIR2 and extends replicative lifespan24. In mouse models, NR prevents high-fat diet-induced weight gain25, fatty liver and diabetic peripheral neuropathy8, noise-induced hearing loss9, heart failure10, and central brain injury12. In addition, oral NR greatly improves survival and hematopoietic stem cell regeneration after irradiation of mice—an activity that was not seen in NA or NAM supplemented mice26. In rats, oral NR promotes resistance to and reversal of chemotherapeutic neuropathy27. In mice, oral NR increases the hepatic levels of the NAD+ metabolome with pharmacokinetics that are superior to that of NA and NAM20. In addition, postpartum female mice and rats who were administered NR exhibited increased lactation and produced offspring that are stronger, less anxious, have better memory, and have enhanced adult hippocampal neurogenesis and body composition as adults13. Because NR does not cause flushing or inhibit sirtuins25 and the genes (NRK1 and NRK2) required for the metabolism of NR to NAD+ are upregulated in conditions of metabolic stress10,28, NR has a particularly strong potential as a distinct vitamin B3 to support human wellness during metabolic stress and aging.
In a variety of animal models, nicotinamide mononucleotide (NMN), the 5′-phosphorylated form of NR, has also shown promise in conditions of metabolic stress and aging29. Moreover, the gut-expressed multispanning membrane protein Slc12a8, previously annotated as a Na+/K+ Cl− transporter, has been proposed to be a specific transporter of nicotinamide mononucleotide (NMN)30. However, the assignment of Slc12a8 as a transporter of NMN occurred without a reliable LC-tandem MS assay for the expected concentration of NMN31 and are inconsistent with genetic, cell biological, and pharmacological evidence from multiple studies demonstrating that NMN is extracellularly converted to NAM and NR prior to intracellular conversion to NMN and the rest of the NAD metabolome12,32,33,34,35,36. While it remains possible that data will emerge showing convincing NMN transport in one or more tissues, the consensus view is that NMN is a usefully circulating metabolite that makes NR available at plasma membranes, which express the 5′-nucleotidase activity of CD731,34. To our knowledge, tests of the safety and human oral availability of NMN are not yet available.
A crystalline form of NR chloride termed NIAGEN has been evaluated in a battery of preclinical studies including a bacterial reverse mutagenesis assay, an in vitro chromosome aberration assay, an in vivo micronucleus assay, and acute, 14-day and 90-day rat toxicology37. In the 90-day toxicology study, NR had a similar toxicity profile to NAM at equimolar doses, the lowest observed adverse effect level (LOAEL) for NR was 1000 mg/kg/day, and the no observed adverse effect level (NOAEL) was 300 mg/kg/day. NIAGEN is Generally Recognized as Safe (GRAS) in the United States for use in food products38 and the subject of two new dietary ingredient notifications39,40, which were filed with the United States Food and Drug Administration without objection.
To date, NR has also been tested in six clinical trials. The first clinical trial of NR established the safe oral availability of single doses and the timecourse by which NR elevates the human blood NAD metabolome20. The second trial provided additional safety data for healthy people taking NR for 8 days41. The third and fourth trials addressed NR safety in healthy people either taking 500 mg NR twice daily for 6 weeks or combination of up to 500 mg NR and 100 mg pterostilbene per day for 8 weeks42,43. Whereas Dellinger et al.42 found that the combination of NR and pterostilbene signficantly elevated low density protein cholesterol (LDL-C) in a dose and time-depended fashion42, no signficant increases in LDL-C were seen following the adminstration of NR alone43. A fifth clinical trial documented the safety and tolerance of ingesting 2 grams NR per day for 12 weeks in obese men and post hoc analyses suggested that there was an improvement in fatty liver in the NR-treated group44. In a sixth clinical trial, single 500 mg doses of NR depressed markers of oxidative damage while increasing NADPH and exercise performance in older individuals45.
To address the dose-dependent oral availability and safety of NR in overweight adults and the safety of daily NR without pterostilbene including effects on LDL-C and blood levels of HCY, we conducted a randomized, 8-week placebo-controlled trial with 3 doses of NR in overweight but otherwise healthy adults. Here we show that once a day doses of NR up to 1 gram per day are safe and orally available. Blood NAD+ was increased in study subjects in a dose-dependent manner with NAD+ levels achieving 14% to 114% increased levels within 2 weeks that were sustained. We also establish that daily high dose ingestion of NR does not elevate LDL-C or plasma HCY.
Methods
Study design
One hundred and forty healthy male and female participants were enrolled in a randomized, double-blind, placebo-controlled parallel study to investigate the safety and effect of NR (100 mg/day, 300 mg/day, and 1000 mg/day) on NAD+ metabolite concentrations in urine and blood over 8 weeks. The study consisted of a 2-week run-in and 8-week supplementation period (Fig. 1). To minimize the effect of dietary influences on NAD+ metabolite levels, subjects were instructed to avoid foods that contain high amounts of tryptophan and forms of vitamin B3 during the run-in and NR supplementation periods. After screening, all subjects attended the clinic prior to the run-in period to review their medical history and health status and receive counseling for the dietary restrictions. At the end of the run-in period (Day 0), the subjects visited the clinic for baseline safety assessments, blood and urine collection, randomization to one of four supplementation groups (placebo, 100 mg, 300 mg, 1000 mg NIAGEN per day groups; n = 35/group), and additional dietary restriction counseling. The subjects were then released to consume their study product for the subsequent 56 days, attending the clinic on Day 7, 14, 28, and 56 for safety assessments, and blood and urine collection.
The study was conducted at KGK Science Inc. Suite 1440, One London Place, 255 Queens Ave, London, Ontario, following Good Clinical Practice (GCP) guidelines and in accordance with the ethical principles that have their origins in the Declaration of Helsinki and its subsequent amendments. The study was reviewed by the Natural Health Product Directorate (NHPD), Health Canada and a research ethics board. Notice of authorization was granted on December 9th, 2015 by the NHPD, Ottawa, Ontario and unconditional approval was granted on February 5th, 2016 by the Institutional Review Board (IRB Services, Aurora, Ontario). The study was registered on clinicaltrials.org on March 18, 2016 as NCT0271593 and posted to the WHO International Clinical Trial Registry Platform on January 3, 2016. External monitoring of source documents was conducted by ClynProject Consulting, LLC.
- Related articles
- Related Qustion
- What are the benefits of Nicotinamide riboside chloride supplement? What is the appropriate daily dosage? May 15, 2025
Nicotinamide riboside chloride (NRCl) is an FDA-approved nutritional supplement that can be used to increase NAD+ levels.
- Niacin vs Niacinamide vs Nicotinamide Riboside: what's the difference? Mar 21, 2024
Nicotinic acid, nicotinamide and nicotinamide riboside are the vitamin B3 precursors of nicotinamide adenine dinucleotide (NAD) in the human diet.
- Nicotinamide riboside chloride: biological activities and preclinical studies Jan 24, 2024
Nicotinamide riboside chloride increases NAD+ levels, has similar toxicity to nicotinamide, and shows potential benefits in metabolism, liver health, and exercise performance.
Telmisartan oral tablet is a prescription drug that’s available as the brand-name drug Micardis. It’s also available as a generic drug. Generic drugs usually cost less than the brand-name version. In some cases, they may not be available in all strengths or forms as the brand-name drug.....
Aug 19,2019APIIf you have atrial fibrillation (a condition in which the heart beats irregularly, increasing the chance of clots forming in the body, and possibly causing strokes) and are taking apixaban to help prevent strokes or serious blood clots, you are at a higher risk of having a stroke after you stop taking this medication. Do not stop taking apixaban without talking to your doctor.....
Aug 19,2019Biochemical EngineeringNicotinamide riboside chloride
23111-00-4You may like
Nicotinamide riboside chloride manufacturers
- Nicotinamide riboside chloride
-
- 2025-09-28
- CAS:23111-00-4
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Nicotinamide riboside chloride
-

- $20.00 / 1kg
- 2025-09-28
- CAS:23111-00-4
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 10000
- Nicotinamide riboside chloride
-

- $0.00 / 1KG
- 2025-09-28
- CAS:23111-00-4
- Min. Order: 1KG
- Purity: 97%min
- Supply Ability: 100KG