Sodium dodecyl sulfate : A very useful surfactant for Scientific Invetigations
Nov 8,2019
Sodium dodecyl sulfate is most researched and best understood widely used anionic surfactant. It is obtained in a state of powder as well as in pellet and used in Polymer Biotechnology and Biochemistry. This review article is related to present and future uses of sodium dodecylsulphate in different applied fields. The critical micelle concentration (cmc) of Sodium dodecylsulphate changes with change in temperature and addition of electrolyte. It causes skin and eye irritation in higher concentration but such effect decreases with decrease in concentration and widely used as cleaning agent and house hold products.
Introduction
The most common sulphate surfactant is sodium dodecylsulphate (SDS) probably most extensively studied anionic surfactant known to science [Warra: 2013]. It is sometimes referred to as sodium laurylsulphate (SLS). It is an organic compound commercially found in the form of Powder or Pellet. It is found that pellet form is more soluble in water and less toxic than the powder form. Like all detergents it removes oils from the skin and can cause skin and eye irritation in higher concentration. If the concentration is less than 1%, it's such carcinogenic character decreases. It's cmc in pure water at 250C is 0.0082M [Mukerjee and Mysels:1971] and the aggregation number at this concentration is usually considered to be about 62 [Turro and Yekta:1978]. If any electrolyte is added to a solution of SDS or temperature or pressure changes, the aggregation behavior of SDS affected. For example, when chloride, acetate and propionate co-ions are added to the aqueous solution of SDS, then the value of cmc affected. Acetate, propionate and butyrate do not have any effect on the cmc of SDS. Butyrate ion, however, shows influence on the adsorption behaviour and aggregation number of SDS. Thus, co-ion with up to four carbon atoms does not affect the cmc of SDS. Further investigation is required to ascertain the minimum number of carbon atoms required in the co-ion for showing the influence on cmc of SDS [Umlong and Ismail : 2006].
As other surfactants sodium dodecylsulphate has an amphiphilic molecule that is contained hydrophilic and hydrophobic moiety. Research showed that SDS is not carcinogenic when either applied directly to skin or consumed [CIR: 1983].The micelle ionization fraction (α) of SDS is around 30%[Barney et.al: 1998]. Molecular weight is 288.5 and micelle molecular weight is 18000g. SDS can be prepared by reacting dodecanol with sulfuric acid and reacting the products with sodium hydroxide [Warra: 2013]. The reactions are given below:
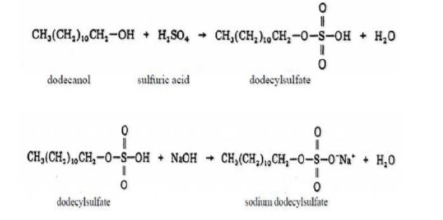
It can also prepared by neutralization of Dodecane (Dodecyl) Sulfuric acid ester with Caustic alkali[Warra: 2013].

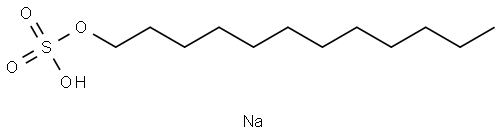
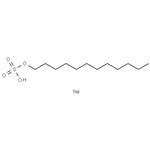
Molecular formula of SDS is C12H 22SO4Na. Structure of SDS can be represented as follows:
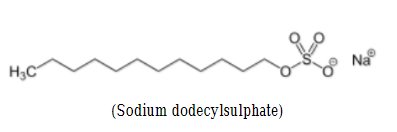
SDS is an organosulfate compound consisting of a 12-carbon chain as tail attached to a sulfate group, having head and tail the amphiphilic properties required for a detergent[ Singh:2012].
It has been found that SDS acts as irritate to skin of the face with for constant exposure to long time (more than an hour) in young adults [Marrakchi and Maibach: 2006]. SDS may cause chronic skin problems in individuals with chronic skin hypersensitivity, such affect is more in some people than others [Agner: 1991; Nassif et.al: 1994; Löffler and Effendy: 1999]. In animal studies SDS appears to cause skin and eye irritation. SDS can contact the face from aerosolized dust during SDS preparation for laboratory purposes. To prevent contact with face and eyes, a dust free “pellet” form of SDS is available which essentially eliminates SDS dust inhalation and contact with the face. Sodium dodecylsulphate is extensively used both for fundamental studies as well as in many industrial applications [Tadros: 2005]. SDS is mainly used in detergents for laundry with many cleaning applications[Smulders et.al: 2002]. SDS is a highly effective surfactant and is used in any task requiring the removal of oily stains and residues[Devesa-Rey et.al: 2011]. Surfactants such as SLS are also found in ointments and creams as well as in cleansers [Chhetri et.al: Healthy Health Products]. Sodium dodecylsulfate is used in household and car cleaning products because of its ability to produce foam, cut through grease, and suspend soil particles in such a way so that they can easily be washed away[Warra: 2013].
Walker et.al:2005 studied the acute and short-term toxicity in rats that have been made on the surfactants sodium lauryl sulphate, sodium lauryl (3EO) ethoxysulphate and their matches C12–C15 alcohol sodium sulphate and C12–C15 alcohol sodium (3EO) ethoxysulphate. The acute oral LD50s of the four materials were found to range from 1 to 2 g/kg. This clearly indicates the toxicity level of sodium lauryl sulfate (SLS). SLS is reported to be a strong oxidizing agent and is a highly toxic compound. This causes respiratory, eye and skin irritation. Carcinogenic nitrates can form in the manufacturing of SLS or by its inter reaction with other nitrogen bearing ingredients which show permanent eye damage in young animals from skin contact in non eye areas. The studies indicated that SLS enters and maintains the residual levels in the heart, the liver, the lungs and the brain from skin contact. This poses a serious health threat by its use in shampoos, cleansers, and tooth pastes. SLS is used in almost all health products including soaps, shampoos, bubble baths, tooth paste, washing up liquid, Laundry detergent, children soaps and shampoos stain remover, carpet cleaner, fabric glue, body wash, shaving cream, mascara, mouth wash, skin cleanser, moisturizing lotion and sun screen.
A clinical study found SLS toothpaste caused a higher frequency of aphthous ulcers than both cocoamidopropyl betaine or a detergent-free paste, on 30 patients with frequent occurrences of such ulcers[Herlofson and Barkvoll:1996]. A clinical study comparing toothpastes with and without SLS found that it had no significant effect on ulcer patterns[ Healy et.al:1999].SDS is commonly used in preparing proteins for electrophoresis in the SDS-PAGE technique[Olędzka et.al:2012]. SDS is a powerful anionic detergent that binds tightly to proteins, denaturing them fully at elevated temperature (100°C) in the presence of a reducing agent like mercaptoethanol. Multi-subunit proteins (whose subunits are noncovalently associated or covalently attached by disulfide bonds) are dissociated into monomeric units by the combined action of SDS and a reducing agent at elevated temperature. So much SDS binds to a protein that the protein’s intrinsic charge is overwhelmed by the bound SDS[Ward and Swiatek: 2009].
Sodium dodecylsulfate is also used in the analysis of hemoglobin [Oshiro et.al:1982]. Sodium dodecylsulfate (SDS) appears more effective than Triton X-100 for removing nuclei from dense tissues and organs such as the kidney and temporomandibular joint while preserving tissue mechanics [Nakayama et.al: 2010; Lumpkins et.al: 2008].
The addition of a detergent such as SDS to a decellularization protocol can make the difference between complete and incomplete cell nuclei removal [Yang et.al: 2010]. Sodium laurylsulfate temporarily diminishes perception of sweetness [Michael: 1985], an effect commonly observed after recent use of toothpaste containing this ingredient [Josh: Discovery Health]. SDS represent an effective microbicide, it can inhibit and prevent infection by various enveloped and non-enveloped viruses such as the Herpes simplex viruses, HIV, and the Semliki Forest Virus[Piret et.al: 2001]. SDS is used in Biotechnology for numerous applications [Angerhofer and Pezzuto: 1993].
- Related articles
- Related Qustion
- Laboratory and medical uses of Sodium dodecyl sulfate (SDS) detergent May 6, 2024
Sodium dodecyl sulfate (SDS), also known as Sodium Lauryl Sulfate (SLS), is an anionic surfactant that functions as a detergent, emulsifier and protein denaturant.
- Sodium dodecyl sulfate: Application, preparation and toxicity Apr 20, 2023
Sodium dodecyl sulfate is an anionic surfactant used in many cleaning and hygiene products. It consists of a 12-carbon tail attached to a sulfate group.
Heptane is a chemical derived from petroleum and a member of the alkane series. It is classified as a “straight-chain” alkane. As a chemical, it is a colorless liquid hydrocarbon.....
Nov 8,2019Chemical ReagentsAmmonium chloride is yielded as a by-product in the ammonia-soda process for making sodium carbonate. It also is produced by reaction of ammonium sulfate and sodium chloride solutions. When mixed with slaked lime (calcium carbonate), ammoni....
Nov 8,2019APISodium dodecyl sulfate
151-21-3You may like
Sodium dodecyl sulfate manufacturers
- Sodium dodecyl sulfate
-
- 2025-07-09
- CAS:151-21-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Sodium dodecyl sulfate
-

- $999.00/ ton
- 2025-07-07
- CAS:151-21-3
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 5000
- Sodium dodecyl sulfate
-

- $100.00 / 1kg
- 2025-07-07
- CAS:151-21-3
- Min. Order: 25kg
- Purity: 99.99%
- Supply Ability: 200ton