The uses of chloroacetyl chloride
Nov 13,2019
Chloroacetyl chloride, a colorless to yellow liquid, is a bi-functional compound that is useful as a chemical building block. This chemical is mainly used in the production of herbicides, in the formulation of active pharmaceutical ingredients, and in the production of other useful chemicals.
Uses
The main use of chloroacetyl chloride is in the manufacturing of herbicides used in agriculture. Especially, popular herbicides such as alachlor and butachlor are manufactured using chloroacetyl chloride. However, due to the fact that such herbicides create environmental pollution, production and use of alachlor and butachlor has been banned in the EU region.
However, several countries in Latin America and in the Asia Pacific region continue the use of such herbicides and therefore the demand of these herbicides is steady and rising. This is expected to boost the growth of the global chloroacetyl chloride market. In case of medical emergencies, the human body is unable to produce the adrenalin hormone. Thus, demand for externally produced adrenalin is expected to increase due to an increasing risk of life threatening diseases. Chloroacetyl chloride is used in the production of epinephrine (adrenalin hormone). This is also expected to fuel the growth of the global chloroacetyl chloride market over the forecast period.
Chloroacetyl chloride occasionally serves as a chlorinating agent. 2,4-Pentanedione was chlorinated at the 1- and 5-positions upon treatment with ClCH2COCl/AlCl3 in the presence of Cu(OAc)2. a,b Chloroacetyl chloride has also been used to transform 2-picoline N-oxide and 2-oxo-2-dimethylamino-5-methyl-1,2-oxophospholane to 2-chloromethylpyridine and 2-oxo-2-chloro-5-methyl-1,2- oxophospholane, respectively.
Reactions
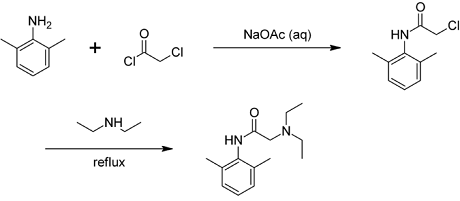
Chloroacetyl chloride is bifunctional—the acyl chloride easily forms esters and amides, while the other end of the molecule is able to form other linkages, e.g. with amines. The use of chloroacetyl chloride in the synthesis of lidocaine is illustrative:
Safety
Like other acyl chlorides, reaction with other protic compounds such as amines, alcohols, and water generates hydrochloric acid, making it a lachrymator.
There is no regulated permissible exposure limit set by the Occupational Safety and Health Administration. However, the National Institute for Occupational Safety and Health has set a recommended exposure limit at 0.05 ppm over an eight-hour work day.
- Related articles
- Related Qustion
- What is the hazard of Chloroacetyl chloride to humans? May 15, 2024
Chloroacetyl chloride is a potent acylating agent. Exposure to Chloroacetyl chloride or its vapours by any route (inhalation, ingestion, dermal contact) is toxic to humans.
- Chloroacetyl chloride: applications in synthesis and toxicology Jul 21, 2023
Chloroacetyl chloride is versatile in organic synthesis, used for anti-tumor drug research, surfactant preparation, but highly toxic and requires caution in handling.
Octamethylcyclotetrasiloxane, also called D4, is an organosilicon compound with the formula [(CH3)2SiO]4. It is a colorless viscous liquid. Octamethylcyclotetrasiloxane is a common cyclomethicone.....
Nov 13,2019Organometallic compoundso-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2. with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration).....
Nov 13,2019Organic Raw MaterialChloroacetyl chloride
79-04-9You may like
Chloroacetyl chloride manufacturers
- Chloroacetyl chloride
-

- $0.00 / 250kg/drum
- 2025-12-18
- CAS:79-04-9
- Min. Order: 250kg/drum
- Purity: 99%
- Supply Ability: 100000MT
- Chloroacetyl chloride
-

- $10.00 / 1KG
- 2025-12-11
- CAS:79-04-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Chloroacetyl chloride
-

- $10.00 / 1KG
- 2025-05-26
- CAS:79-04-9
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 10 ton