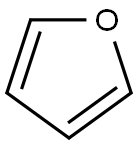
Toxicity and hazards of Furan
Oct 27,2021
Furan occurs naturally in oils distilled from rosin containing pinewood. In addition, many natural foods contain the furan ring structure and substituted furans may be formed through cooking of simple carbohydrates. Furan is also found in tobacco smoke as well as wood smoke and gas emissions from gasoline and diesel engines. Furan has also been detected in industrial effluents and can be emitted to the air from petroleum refineries and coal-mining and gasification plants.
Furan is a solvent used in the organic synthesis of pyrrole, tetrahydrofuran, and thiophene. It is also used as a solvent for resins and in the production of lacquers, agricultural chemicals, and stabilizers.
Toxicity and hazards
Furan can cause eye, skin, and mucus membrane irritation, burning sensation and, in severe cases, corrosion. If inhaled, furan may produce pulmonary edema and bronchiolar necrosis. When absorbed, furan can cause central nervous system (CNS) depression to the point of narcosis and tonic seizures.
Furan is metabolized by the cytochrome P450 enzymes in the liver and other tissues. Furan is metabolized to cis-2- butene-1,4-dial. This active metabolite is acutely toxic to liver cells. The furan ring undergoes oxidative cleavage and forms highly reactive furan radical cations or epoxides, which react directly with cellular nucleophiles. These reactive metabolites may react directly with deoxyribonucleic acid (DNA) or with cellular proteins to produce disruption of cellular functions and cell death. Chronic cell death and regeneration produced by chronic furan exposure may be a significant factor in the carcinogenicity potential of the chemical. In addition, there is some evidence to suggest that the reactive metabolites of furan may induce mutations in cellular genes.
Furan may be released to the environment as a waste industrial product or from unintentional or accidental releases. If released to soil, it is expected to volatilize. If released to water, furan is not expected to adsorb to suspended particles and sediment and is likely to volatilize to ambient air. Sulfate-reducing bacteria can degrade furan. However, under nonsulfate-reducing conditions, biodegradation in soil and water is expected to be slow. In the air, furan will exist as a vapor and will be subject to degradation by reacting with hydroxyl radicals.
- Related articles
- Related Qustion
- Electrophilic Reactions of Furan Jan 24, 2022
Furans are fairly soluble in organic solvents, including alcohol and ether, but slightly soluble in water. They are toxic and may be carcinogenic in humans. The parent furan has a bp of 31.5°C. The ionization potential is 8.89 eV and the di
- Uses of Furan Jan 24, 2022
Furan is a five-membered aromatic oxygen heterocycle, comprised of four conjugated carbon atoms and one oxygen atom in a cyclic system. Furan is a planar aromatic compound because one of the two lone pairs of electrons on the oxygen partici
Formic acid is found in nature as it is produced by plants, insects, and bacteria. However, it is also used in industries for the manufacture of numerous consumer products.....
Oct 27,2021Organic reagentsNavitoclax, also known as ABT-263 and ABT 263, is a potent, oral biologically active inhibitor of the Bcl-2 protein family, acting on Bcl-xL, Bcl-2 and Bcl-w, with Ki of ≤0.5 nM, respectively , ≤1 nM and ≤1 nM.....
Oct 28,2021InhibitorsFuran
110-00-9You may like
- Furan
-
- $1.10 / 1g
- 2023-07-27
- CAS:110-00-9
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
- Furan
-

- $200.00 / 1kg
- 2023-06-26
- CAS:110-00-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
- Furan
-

- $200.00 / 1kg
- 2023-05-27
- CAS:110-00-9
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 50000 tons