Trimethylaluminium-Health Hazards and Toxicity
Sep 10,2019
Trimethylaluminum is called TMA for short. Trimethylaluminum was prepared by Buckton for the first time using methyl mercury and aluminum in 1865, but until the 1950s, TMA applications has been still limited to the scope of laboratory, its research and application was far less active than organic magnesium and organic lithium for a long time. In recent years, with the development of the research of polyolefins, TMA is as one of the cocatalyst, and starting raw materials for synthesis of cocatalyst catalysts methylalumoxane (of MAO) and modified methylaluminoxane (MMAO) in metallocene catalyst systems. In the organic chemical industry, especially polymer chemical industry, its importance began to appear.
Toxicity Data
TLV-TWA (ACGIH) 2 mg (Al)/m3
Major Hazards
Highly reactive, pyrophoric substances; corrosive on contact with skin and eyes.
Toxicity
Trimethylaluminum and related alkylaluminum reagents are pyrophoric materials that can react explosively with the moisture in tissues, causing severe burns. The heat of reaction can also ignite the methane gas generated, resulting in thermal burns. Alkylaluminum reagents are corrosive substances, and contact is extremely destructive to the eyes, skin, and mucous membranes. Inhalation of trimethylaluminum and other volatile alkylaluminum compounds may cause severe damage to the respiratory tract and can lead to fatal pulmonary edema.
Flammability and Explosibility
Trimethylaluminum is pyrophoric and burns violently on contact with air or water. Other alkylaluminum reagents show similar behavior, although most are not as volatile as trimethylaluminum. Water or CO2 fire extinguishers must not be used to put out fires involving trialkylaluminum reagents. Instead, dry chemical powders such as bicarbonate, Met-L-X®, or inert smothering agents such as sand or graphite should be used to extinguish fires involving trialkylaluminum compounds.
Reactivity and Incompatibility
Trialkylaluminum reagents are highly reactive reducing and alkylating agents. They react violently with air, water, alcohols, halogenated hydrocarbons, and oxidizing agents. These reagents are often supplied as solutions in hydrocarbon solvents, which are less hazardous than the pure liquids.
Storage and Handling
Trialkylaluminum agents should be handled in the laboratory using the "basic prudent practices", supplemented by the additional precautions for work with highly flammable and reactive substances. Safety glasses, impermeable gloves, and a fire-retardant laboratory coat should be worn at all times when working with these compounds. Trialkylaluminum reagents should be handled only under an inert atmosphere.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If trialkylaluminum compounds are ingested, obtain medical attention immediately. If any of these compounds are inhaled, move the person to fresh air and seek medical attention at once.
Any spill of trialkylaluminum will likely result in fire. Remove all ignition sources, put out the trialkylaluminum fire with a dry chemical extinguisher, sweep up the resulting solid, place in an appropriate container under an inert atmosphere, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess trialkylaluminum reagents and waste material containing these substances should be placed in an appropriate container under an inert atmosphere, clearly labeled, and handled according to your institution's waste disposal guidelines. Alternately, small quantities of trialkylaluminum reagents can be destroyed in the laboratory by experienced personnel by slow addition of t-butanol to a solution of the reagent in an inert solvent such as toluene under an inert atmosphere such as argon. The resulting mixture should then be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
- Related articles
- Related Qustion
Trifluoroacetic acid is a highly corrosive substance. Contact of the liquid with the skin, eyes, and mucous membranes can cause severe burns, and ingestion can result in serious damage to the digestive tract.....
Sep 10,2019Organic ChemistryBrigatinib (marketed as Alunbrig) is a small-molecule targeted cancer therapy being developed by ARIAD Pharmaceuticals, Inc. Brigatinib acts as both a anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor. B....
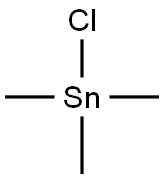
Sep 11,2019APITRIMETHYLTIN CHLORIDE
1066-45-1You may like
TRIMETHYLTIN CHLORIDE manufacturers
- TRIMETHYLTIN CHLORIDE
-
- $0.00 / 25kg
- 2025-12-01
- CAS:1066-45-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- TRIMETHYLTIN CHLORIDE
-

- $1.00 / 1KG
- 2019-12-20
- CAS:1066-45-1
- Min. Order: 1KG
- Purity: 95%~99%
- Supply Ability: 1ton