Voriconazole: the newest triazole antifungal agent
Oct 28,2019
Voriconazole is the newest agent in the armamentarium against fungal infections. It is a triazole antifungal with a structure related to that of fluconazole and a spectrum of activity comparable to that of itraconazole. Voriconazole was approved by the Food and Drug Administration in May 2002 for the treatment of invasive aspergillosis and refractory infections of Scedosporium apiospermum and Fusarium spp. Studies have also shown it to be a promising agent for empiric treatment in febrile neutropenia.
INDICATIONS
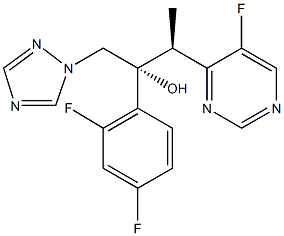
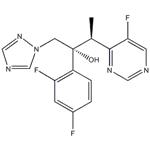
Voriconazole (VFEND, Pfizer Ireland Pharmaceuticals, Ringaskiddy, Ireland) is a triazole antifungal agent that inhibits fungal ergosterol biosynthesis (5). It is structurally related to fluconazole, with the major difference being the substitution of a fluoropyrimidine grouping in place of a triazole moiety (5, 6). Voriconazole is indicated for the treatment of invasive aspergillosis. It is also indicated for the treatment of fungal infections caused by S. apiospermum or Fusarium spp. that are refractory to other antifungal agents (5).
PHARMACOLOGY
Like the other triazole antifungals, voriconazole exerts its antifungal activity by inhibition of 14-alpha-lanosterol demethylation, which is mediated by fungal cytochrome P450 enzymes (2, 5, 6). This inhibition is more selective for fungal than for mammalian enzyme systems. The accumulation of 14-alphamethyl sterols results in a decrease in ergosterol, which is an essential component of fungal cell wall formation. The resulting cell wall abnormalities are thought to be responsible for voriconazole's antifungal activity.
PHARMACOKINETICS
The pharmacokinetic profile of voriconazole has been defined from various studies in healthy volunteers, patients, and special populations (5, 7–9). Voriconazole is unique because of its saturable metabolism, resulting in a nonlinear Michaelis-Menten pharmacokinetic profile. Thus, when the dosage of voriconazole is increased, a larger-than-proportional increase is seen in drug exposure. This also results in a variable elimination half-life (from 6 to 24 hours) depending on the dosage of voriconazole given.
The pharmacokinetic properties of voriconazole are similar whether given intravenously or orally. It is well absorbed, with an oral bioavailability of > 95%. It takes 1 to 2 hours to reach maximum concentrations after dosing. However, the bio- availability is decreased and the time to maximum concentration extended when voriconazole is administered with a high-fat meal (2, 5). Absorption is not diminished when voriconazole is administered with gastric acid–suppressing agents such as cimetidine, ranitidine, or omeprazole.
Voriconazole is extensively distributed into tissue, with a volume of distribution of approximately 4.6 L/kg (5). A case study reports that cerebrospinal concentrations were 42% to 67% of plasma concentrations in 2 patients with acute leukemia who had Aspergillus spp. meningitis (10). Protein binding is approximately 58% in plasma and is independent of various plasma drug concentrations reached after single and multiple oral doses.
Voriconazole is extensively metabolized in the liver to the N-oxide metabolite (5, 8, 10). The main hepatic cytochrome P450 enzyme responsible for voriconazole's metabolism is CYP2C19, although CYP2C9 and CYP3A4 are also involved. Studies performed in vitro suggest that voriconazole, as well as its metabolite, inhibits these enzymes as well. CYP2C19 is subject to genetic polymorphism, leaving certain populations susceptible to decreased metabolism and increased plasma levels of voriconazole. Persons of Asian descent have up to a 20% chance of being a poor metabolizer, while Caucasian and African American individuals have up to a 5% chance (11). Studies have indicated that poor metabolizers can have an area under the curve up to 4 times higher than that of homozygous extensive metabolizers and 2 times higher than that of heterozygous extensive metabolizers. Poor metabolizers also have higher plasma accumulation after multiple dosing.
SPECTRUM OF ACTIVITY
Voriconazole has shown activity against Aspergillus spp. in vitro (8, 12–16). Growth-inhibition studies have shown voriconazole to be fungicidal against the various Aspergillus spp. Voriconazole and itraconazole have similar minimum inhibitory concentrations (MICs) when tested against Aspergillus spp. Voriconazole demonstrated low MICs for all Aspergillus spp. tested but appeared to be most active against Aspergillus fumigatus. Abraham and colleagues also found that voriconazole maintains activity against itraconazole-resistant A. fumigatus isolates (17). This suggests that voriconazole may have alternate mechanisms of fungal killing since complete cross-resistance did not develop
- Related articles
- Related Qustion
- Is Voriconazole safety? Jun 18, 2024
Voriconazole was approved by the US Food and Drug Administration (FDA) in May 2002.
- Voriconazole:azole drug Mar 29, 2022
Voriconazole (Vfend, UK-109,496) is a synthetic triazole derivative with potent broad-spectrum activity. Its structure is similar to fluconazole, but one triazole ring is replaced with a fluorinated pyrimidine
Food and Drug Administration (FDA) a New Drug Application (NDA) for UX007 (triheptanoin) for the treatment of long-chain fatty acid oxidation disorders (LC-FAOD), a group of genetic disorders in which the body is unable to convert long-chai....
Oct 28,2019Organic Raw MaterialRucaparib is the generic name for the trade name drug Rubraca?. In some cases, health care professionals my use the trade name Rubraca? when referring to the generic drug name rucaparib.....
Oct 29,2019APIVoriconazole
137234-62-9You may like
- Voriconazole
-

- $0.00 / 1kg
- 2025-10-29
- CAS:137234-62-9
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000
- Voriconazole
-

- $0.00 / 1kg
- 2025-10-29
- CAS:137234-62-9
- Min. Order: 1kg
- Purity: 98.5%min
- Supply Ability: 100kg
- Voriconazole
-
- 2025-10-28
- CAS:137234-62-9
- Min. Order:
- Purity: 0.99
- Supply Ability: