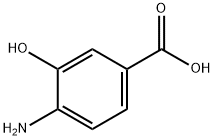
What is 4-Amino-3-hydroxybenzoic acid?
Jan 16,2020
4-Amino-3-hydroxybenzoic acid is used in the preparation of various pharmaceutical compounds such as sphingosine kinase inhibitors. Store at 4℃. Store in cool, dry conditions in well-sealed containers. Keep container tightly closed. Store away from strong oxidizing agents.
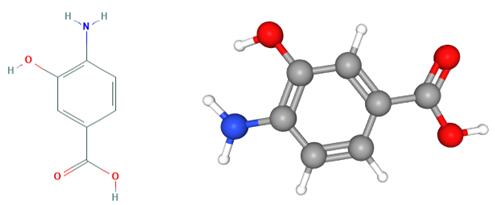
Fig 1. Chemical structure formula and three-dimensional structure of 4-Amino-3-hydroxybenzoic acid
4-Amino-3-hydroxybenzoic acid is used in the preparation of various pharmaceutical compounds such as sphingosine kinase inhibitors[1].Bordetella sp. strain 10d metabolizes 4-amino-3-hydroxybenzoic acid via 2-hydroxymuconic 6-semialdehyde. Cell extracts from 4-amino-3-hydroxybenzoate-grown cells showed high NAD+-dependent 2-hydroxymuconic 6-semialdehyde dehydrogenase, 4-oxalocrotonate tautomerase, 4-oxalocrotonate decarboxylase, and 2-oxopent-4-enoate hydratase activities, but no 2-hydroxymuconic 6-semialdehyde hydrolase activity. These enzymes involved in 4-amino-3-hydroxybenzoate metabolism were purified and characterized. When 2-hydroxymuconic 6-semialdehyde was used as substrate in a reaction mixture containing NAD+ and cell extracts from 4-amino-3-hydroxybenzoate-grown cells, 4-oxalocrotonic acid, 2-oxopent-4-enoic acid, and 4-hydroxy-2-oxovaleric acid were identified as intermediates, and pyruvic acid was identified as the final product. A complete pathway for the metabolism of 4-amino-3-hydroxybenzoic acid in strain 10d is proposed. Strain 10d metabolized 2-hydroxymuconic 6-semialdehyde derived from 4-amino-3-hydroxybenzoic acid via a dehydrogenative route, not via a hydrolytic route. This proposed metabolic pathway differs considerably from the modified meta-cleavage pathway of 2-aminophenol and those previously reported for methyl- and chloro-derivatives.
2-Amino-5-carboxymuconic 6-semialdehyde is an unstable intermediate in the meta-cleavage pathway of 4-amino3-hydroxybenzoic acid in Bordetella sp. strain 10d. In vitro, this compound is nonenzymatically converted to 2,5-pyridinedicarboxylic acid. Crude extracts of strain 10d grown on 4-amino-3-hydroxybenzoic acid converted 2-amino-5-carboxymuconic 6-semialdehyde formed from 4-amino-3-hydroxybenzoic acid by the first enzyme in the pathway,4-amino-3-hydroxybenzoate 2,3-dioxygenase, to a yellow compound[3].
References
[1]Chika Orii; Shinji Takenaka; Shuichiro Murakami; and Kenji Aoki . A novel coupled enzyme assay reveals an enzyme responsible for the deamination of a chemically unstable intermediate in the metabolic pathway of 4-amino-3-hydroxybenzoic acid in Bordetella sp. strain 10d. Eur. J. Biochem. 2004, 271,(15), 3248-3254.
[2]Orii C , Takenaka S , Murakami S , et al. Metabolism of 4-amino-3-hydroxybenzoic acid by Bordetella sp. strain 10d: A different modified meta-cleavage pathway for 2-aminophenols.[J]. Bioscience Biotechnology & Biochemistry, 2006, 70(11):2653-2661.
[3]Orii C . A novel coupled enzyme assay reveals an enzyme responsible for the deamination of a chemically unstable intermediate in the metabolic pathway of 4‐amino‐3‐hydroxybenzoic acid in Bordetella sp. strain 10d[J]. European Journal of Biochemistry, 2010, 271(15):3248-3254.
[4]https://pubchem.ncbi.nlm.nih.gov/compound/137566
[5]https://www.chemspider.com/Chemical-Structure.121227.html?rid=02512d5c-ee63-45e3-83e6-6355c54e11db
- Related articles
- Related Qustion
- 4-Amino-3-hydroxybenzoic acid:a fundamental building block Dec 15, 2023
4-Amino-3-hydroxybenzoic acid, a versatile compound, pivotal in drug synthesis, organic construction, microbial metabolism, and dye production.
1'-Acetonaphthones are used as ingredients of fragrances. Their derivatives are used as anti-tubercular agents. The end applications include soap, detergent, beauty care product, household product.....
Jan 16,2020Organic Raw MaterialDimethyl chlorothiophosphate is a chemical that is used as an intermediate in the manufacture of pesticides and plasticisers. It is an organophosphate with sulfur and chlorine also bonded to the central phosphorus atom.....
Jan 16,2020Nucleoside Drugs4-Amino-3-hydroxybenzoic acid
2374-03-0You may like
- Synthesis of furfurylamine
May 18, 2023
- Application of Fmoc-N-Me-Arg(pbf)-OH
Dec 9, 2022
- The Benefits of L-Tyrosine
Nov 22, 2022
4-Amino-3-hydroxybenzoic acid manufacturers
- 4-Amino-3-hydroxybenzoic acid
-

- $0.00 / 1KG
- 2024-04-26
- CAS:2374-03-0
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 100KG
- 4-Amino-3-Hydroxy Benzoic Acid
-
- $20.00 / 1KG
- 2024-04-26
- CAS:2374-03-0
- Min. Order: 1KG
- Purity: 98% & 99%
- Supply Ability: 100MT/year
- 4-Amino-3-hydroxybenzoic acid
-

- $5.00 / 25kg
- 2024-04-25
- CAS:2374-03-0
- Min. Order: 1kg
- Purity: 99.92%
- Supply Ability: 50000tons