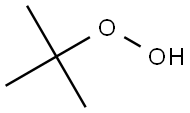
tert-Butyl hydroperoxide-Health Hazard and Toxicity
Sep 6,2019
Description
tert-Butyl hydroperoxide (tBuOOH) is an organic peroxide widely used in a variety of oxidation processes, for example Sharpless epoxidation.[3] It is normally supplied as a 69–70% aqueous solution.
Toxicity Data
LD50 oral (rat) 406 mg/kg
LD50 skin (rabbit) 460 mg/kg
LC50 inhal (rat) 500 ppm (4 h)
Health Hazard
tert-Butyl hydroperoxide is a strong irritant.Floyd and Stockinger (1958) observed thatdirect cutaneous application in rats did notcause immediate discomfort, but the delayedaction was severe. The symptoms were erythemaand edema within 2–3 days. Exposureto 500 mg in 24 hours produced asevere effect on rabbit skin, while a rinse of150 mg/min was severe to eyes.
Toxicity
Moderately toxic by inhalation and ingestion and severely irritating to the eyes and skin. t-Butyl hydroperoxide has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans.
Flammability and Explosibility
tert-Butyl hydroperoxide is a flammable liquid and a highly reactive oxidizing agent. Pure TBHP is shock sensitive and may explode on heating. Carbon dioxide or dry chemical extinguishers should be used for fires involving tert-butyl hydroperoxide.
Reactivity and Incompatibility
tert-Butyl hydroperoxide and concentrated aqueous solutions of TBHP react violently with traces of acid and the salts of certain metals, including, in particular, manganese, iron, and cobalt. Mixing anhydrous tert-butyl hydroperoxide with organic and readily oxidized substances can cause ignition and explosion. TBHP can initiate polymerization of certain olefins.
Storage and Handling
tert-Butyl hydroperoxide should be handled in the laboratory using the "basic prudent practices" described in Chapter 5.C supplemented by the additional precautions for work with reactive and explosive substances (Chapter 5.G). In particular, tert-butyl hydroperoxide should be stored in the dark at room temperature (do not refrigerate) separately from oxidizable compounds, flammable substances, and acids. Reactions involving this substance should be carried out behind a safety shield.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If tert butyl hydroperoxide is inhaled or ingested, obtain medical attention immediately.
Disposal
Excess tert-butyl hydroperoxide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
- Related articles
- Related Qustion
Benzene is a colorless, volatile, highly flammable liquid that is used extensively in the chemical industry and received wide interest in the early days of organic chemistry.Because of its structure, benzene is a very stable organic compoun....
Sep 6,2019Organic Chemistryn-Butyllithium (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a str....
Sep 6,2019Organic Chemistrytert-Butyl hydroperoxide
75-91-2You may like
tert-Butyl hydroperoxide manufacturers
- Tert-Butyl Hydroperoxide
-

- $80.00 / 25L
- 2025-07-16
- CAS:75-91-2
- Min. Order: 1000L
- Purity: 0.7
- Supply Ability: 5000 Metric Ton/Metric Tons per Year
- tert-Butyl hydroperoxide
-
- $10.00 / 1KG
- 2025-06-24
- CAS:75-91-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- T-Butyl Hydro Peroxide
-

- $10.00 / 1kg
- 2025-05-26
- CAS:75-91-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 10 ton