Active Pharmaceutical Ingredients (API), popularly speaking, are the raw materials of medicines, only pharmaceutical raw materials are processed into pharmaceutical preparations , can they become medicines available for clinical use, so drugs we usually eat are the finished drugs through processing. Active Pharmaceutical Ingredients based on its sources can be divided into two major categories ,including chemical synthetic drugs and natural chemical drugs. Chemical synthetic drugs can be divided into organic synthetic drugs and inorganic synthetic drugs. Inorganic synthetic drugs are inorganic compounds ( very few is element), such as aluminum hydroxide, magnesium trisilicate which are used for the treatment of gastric and duodenal ulcers ; organic synthetic drugs are mainly composed of drugs made by basic organic chemical raw materials, through a series of organic chemical reactions (such as aspirin, chloramphenicol, caffeine, etc.). Natural chemical drugs ,based on its sources,can be divided into two categories including biochemical drugs and plant chemical drugs. Antibiotics are generally made by the microbial fermentation, which belongs to the biochemistry category. A variety of semi-synthetic antibiotics occurs in recent years,which are biosynthesis and chemical synthesis combining products.Among active Pharmaceutical Ingredients, the organic synthetic drugs varieties, yields and values have the largest proportion,which are the main pillars of the chemical and pharmaceutical industries. The quality of active Pharmaceutical Ingredients decides whether the formulation is good or bad , so its quality standards are very strict ,countries in the world have developed national pharmacopoeia standards and strict quality control methods for its widely used active Pharmaceutical ingredients.
2-C-Methyl-D-ribono-1,4-lactone: properties, applications and safety
2-C-Methyl-D-ribono-1,4-lactone is a valuable organic compound with moderate hydrophobicity and safe handling. It has diverse applications in pharmaceuticals, synthesis, and biochemistry.
Dec 15,2023 APIN,N'-bis-boc-1-guanylpyrazole: properties, applications and safety
N,N'-Bis-Boc-1-guanylpyrazole is a reactive compound used in organic synthesis and drug discovery, but requires cautious handling due to limited safety information.
Dec 15,2023 APIThe uses and side effect of Diclofenac sodium
Diclofenac sodium is an NSAID that has generally been used due to its high specificity for the arachidonic acid-degrading enzyme COX-2, rather than its isoform COX-1.
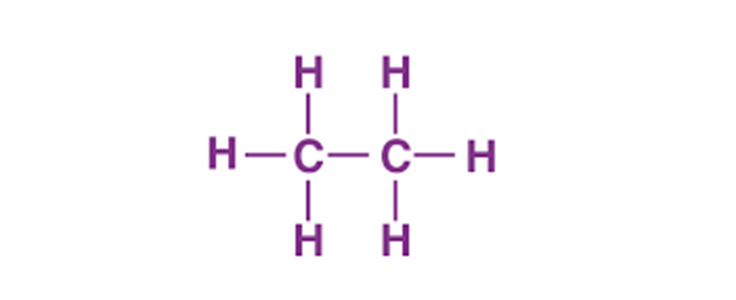
Dec 14,2023 APIC2H6 lewis structure: Hybridization and Molecular Geometry
The Lewis structure of C2H6 (ethane) consists of two carbon atoms and six hydrogen atoms (H)? at a bond angle of 109.5 degrees.
Dec 14,2023 APIHow the N3-lewis structure is formed?
The Lewis structure of N3- (azide ion) is composed of three nitrogen atoms (N).
Dec 14,2023 APIHow to increase the solubility of terbinafine hydrochloride
The poor water solubility of terbinafine hydrochloride leads to reduced oral bioavailability, so attempts were made to improve the solubility of terbinafine hydrochloride by using hydrotropic solubili
Dec 14,2023 APIMethyl 5-chloro-6-methylpyrazine-2-carboxylate: properties, synthesis and safety
Methyl 5-chloro-6-methylpyrazine-2-carboxylate shows potential as an immunomodulator for diseases like cancer, but safety measures are essential when handling.
Dec 14,2023 API2,2'-Bipyridyl-5,5'-dialdehyde: properties, synthesis and safety
2,2'-Bipyridyl-5,5'-dialdehyde is a versatile compound used in catalysis, and materials science due to its unique properties. Proper handling is required due to its irritant classification.
Dec 14,2023 API3-Hydroxypyrazine-2-carboxamide: activities, applications and safety
3-Hydroxypyrazine-2-carboxamide has potential as an anti-aging supplement, treatment for medical conditions, and antiviral agent, but caution is required due to side effects and irritant properties.
Dec 14,2023 API2-Phenyl-2-propyl benzodithioate: properties, synthesis and safety
Versatile 2-phenyl-2-propyl benzodithioate is a crucial chemical intermediate in controlled polymerization with unique properties and potential risks.
Dec 14,2023 API